Press release
Pediatric Epilepsy Therapeutics Market, Advancements in Treatment and Future Outlook for Young Patients
The pediatric epilepsy therapeutics market is a critical segment within the broader healthcare landscape, addressing the urgent need for effective treatments for children suffering from epilepsy. Epilepsy is one of the most common neurological disorders, affecting a significant portion of the pediatric population worldwide. This disorder can cause a wide range of symptoms, from mild seizures to severe neurological impairments, which in turn can significantly impact a child's quality of life and developmental progress. As awareness about pediatric epilepsy grows, the demand for therapeutics continues to rise, creating vast opportunities for both pharmaceutical companies and healthcare providers.Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/12808
The pediatric epilepsy therapeutics market has witnessed robust growth in recent years. With advancements in drug development and new treatment protocols, a surge in market demand is anticipated. The market size is expected to continue expanding, fueled by increasing awareness, enhanced healthcare infrastructure, and the growing incidence of epilepsy in children. Market statistics show a significant year-on-year growth rate, with new drug approvals and novel therapies set to further propel the market. The market is also bolstered by government funding and research initiatives aimed at improving epilepsy care for children. Leading segments within this market include anti-epileptic drugs (AEDs) and innovative therapies targeting drug-resistant epilepsy, with the North American region emerging as the dominant geographical segment due to its advanced healthcare infrastructure and strong market presence of key players.
Key Highlights from the Report:
✦ Increasing incidence of pediatric epilepsy driving the growth of therapeutics.
✦ Rise in demand for personalized medicine for epilepsy management in children.
✦ Significant advancements in AED formulations and delivery systems.
✦ Emergence of innovative therapies for drug-resistant pediatric epilepsy.
✦ Increasing focus on improving quality of life for pediatric epilepsy patients.
✦ Ongoing research initiatives and collaborations to enhance treatment outcomes.
Market Segmentation
The pediatric epilepsy therapeutics market is broadly segmented based on product type, end-user, and geography. By product type, the market is primarily divided into anti-epileptic drugs (AEDs) and other therapeutic interventions such as neuromodulation and surgical therapies. AEDs remain the leading therapeutic option for managing epilepsy in children, with newer formulations such as extended-release tablets and oral solutions gaining traction. These innovations aim to improve patient compliance and therapeutic outcomes.
In addition to AEDs, the market also includes non-pharmacological treatments such as brain stimulation therapies, which have shown promise in addressing cases of drug-resistant epilepsy. These interventions are often considered for children who do not respond well to conventional drug therapies.
The end-user segment of the market includes hospitals, clinics, and homecare settings. While hospitals and clinics account for the majority of the therapeutic interventions, there is a noticeable shift toward homecare options, as they provide more comfort and less disruption to the child's daily life. The growing acceptance of homecare options is particularly important in regions with advanced healthcare systems, where families prefer treatment continuity in a familiar environment.
Regional Insights
Geographically, North America currently dominates the pediatric epilepsy therapeutics market, driven by factors such as well-established healthcare infrastructure, a high incidence of pediatric epilepsy, and the presence of major pharmaceutical companies developing new therapeutic options. The U.S. holds a substantial share of the market, owing to its robust healthcare system and increasing awareness about pediatric epilepsy.
In Europe, the market is also witnessing steady growth, with increasing investments in healthcare technology and pharmaceutical innovations. The pediatric epilepsy therapeutics market in this region is driven by collaborations between academic institutions and healthcare providers, focusing on improving treatment strategies and reducing the burden of the disease on children.
Meanwhile, the Asia Pacific region is emerging as a fast-growing market due to a rising awareness of epilepsy, especially in developing countries such as India and China, where the pediatric population is vast. Increased government healthcare initiatives and improvements in treatment accessibility are expected to contribute significantly to the growth of the market in this region.
Market Drivers
The growth of the pediatric epilepsy therapeutics market is being significantly driven by an increase in the number of pediatric epilepsy cases worldwide. Rising awareness of epilepsy and its early diagnosis is prompting more parents and healthcare providers to seek out effective treatment options. In addition, there has been a surge in the research and development of innovative therapies specifically designed for pediatric patients, which is helping to address gaps in the existing therapeutic landscape.
Another key driver is the increasing demand for personalized medicine in the management of pediatric epilepsy. With advances in genetic testing and biomarker identification, therapies are becoming more tailored to individual patients, improving the efficacy of treatments and minimizing side effects. Furthermore, there is growing support from government agencies and private entities, funding research initiatives focused on finding cures and improving treatment outcomes for pediatric epilepsy patients.
Market Restraints
Despite the promising growth of the pediatric epilepsy therapeutics market, certain restraints could hinder its expansion. One significant challenge is the high cost of developing new pediatric-specific anti-epileptic drugs (AEDs) and treatments. The lengthy clinical trials and regulatory approval processes for pediatric drugs also contribute to the financial burden on pharmaceutical companies, slowing the pace of innovation.
Additionally, the availability of generic AEDs can restrict the growth of more expensive, novel treatments. Parents may often prefer generic medications due to their lower cost, which could limit the adoption of innovative therapies, even if they are more effective. Furthermore, a lack of sufficient healthcare infrastructure in developing regions can make it difficult for pediatric patients to access the treatments they need, stalling market growth in these areas.
Market Opportunities
The pediatric epilepsy therapeutics market presents significant opportunities, particularly in the development of new therapies for drug-resistant epilepsy in children. As many pediatric patients do not respond to traditional AEDs, there is an increasing demand for alternative treatments such as brain stimulation and surgical interventions. These emerging therapies represent untapped opportunities for market players to expand their product offerings and improve patient outcomes.
Moreover, the growing acceptance of homecare treatments and telemedicine in managing pediatric epilepsy presents a new market frontier. By providing children with access to ongoing care in the comfort of their homes, the quality of life for both the patient and their families can be improved. This trend is likely to continue as healthcare systems globally embrace remote patient monitoring and management.
Frequently Asked Questions
How Big is the Pediatric Epilepsy Therapeutics Market?
Who are the Key Players in the Global Pediatric Epilepsy Therapeutics Market?
What is the Projected Growth Rate of the Pediatric Epilepsy Therapeutics Market?
What is the Market Forecast for Pediatric Epilepsy Therapeutics by 2032?
Which Region is Estimated to Dominate the Pediatric Epilepsy Therapeutics Market during the Forecast Period?
Company Insights
• Eisai Co., Ltd.
• GW Pharmaceuticals
• UCB Pharma
• Zogenix, Inc.
• Lupin Pharmaceuticals
• Novartis AG
Recent Developments:
GW Pharmaceuticals received FDA approval for a-based drug for treating pediatric epilepsy in 2020.
UCB Pharma expanded its portfolio of pediatric epilepsy treatments with the launch of a new AED designed for young children in 2021.
Conclusion
In conclusion, the pediatric epilepsy therapeutics market is witnessing robust growth driven by an increasing incidence of epilepsy among children, growing awareness, and advancements in drug development. With new therapies entering the market, including those for drug-resistant epilepsy, and significant regional trends emerging, the market presents both opportunities and challenges for healthcare providers and pharmaceutical companies. By continuing to innovate and focus on patient-specific treatments, stakeholders can capitalize on the increasing demand for effective and personalized pediatric epilepsy care. As the market continues to evolve, it holds immense potential to improve the lives of countless children affected by this debilitating condition.
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web:
https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pediatric Epilepsy Therapeutics Market, Advancements in Treatment and Future Outlook for Young Patients here
News-ID: 3978076 • Views: …
More Releases from Persistence Market Research

Cryogenic Storage Tanks Market Predicted to Hit US$ 12.8 Billion by 2033 Driven …
According to the latest study by Persistence Market Research, the global cryogenic storage tanks market is likely to be valued at US$ 8.6 billion in 2026 and is projected to reach US$ 12.8 billion by 2033, expanding at a CAGR of 5.8% during the forecast period 2026-2033. Rising demand for liquefied gases across energy, healthcare, food processing, and industrial manufacturing sectors is emerging as a key driver shaping the market's…
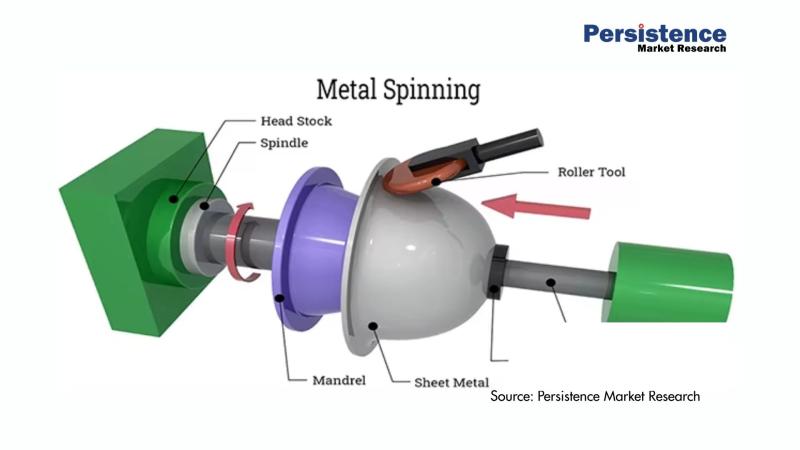
Metal Spinning Products Market Projected to Grow to US$ 4.0 billion by 2033 - Pe …
The global metal spinning products market is poised for substantial growth in the coming years. According to a recent study by Persistence Market Research, the market size is anticipated to reach US$ 4.0 billion by 2033, growing at a robust compound annual growth rate (CAGR) of 4.2% from its current valuation of US$ 3.0 billion in 2026. Metal spinning, a process of shaping metal into precise and symmetrical shapes, is…
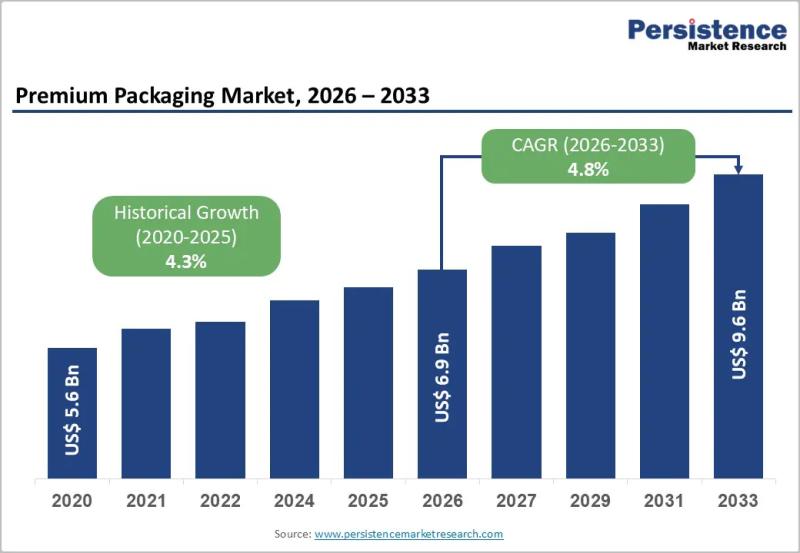
Premium Packaging Market Size Worth US$9.6 Billion by 2033 - Persistence Market …
The premium packaging market has evolved into a critical strategic element for brand differentiation across multiple high value consumer industries. Premium packaging goes beyond basic containment and protection to deliver enhanced aesthetics tactile appeal storytelling and emotional connection. Brands increasingly view packaging as an extension of their identity and a powerful marketing tool that influences purchasing decisions at the point of sale and during the unboxing experience. This shift is…
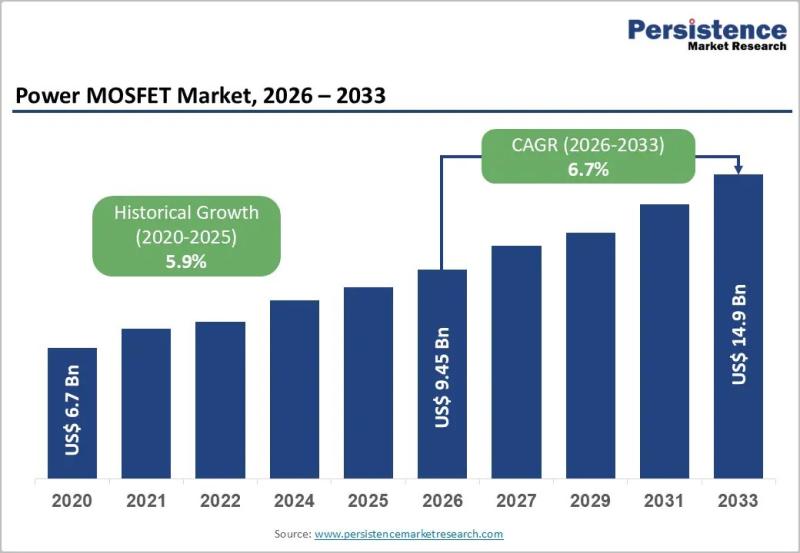
Power MOSFET Market Growth Driven by EVs Renewable Energy and Smart Automation
The global Power MOSFET market is entering a phase of sustained expansion, driven by the accelerating need for energy-efficient and high-performance power management components across industries. In 2026, the market is expected to be valued at US$ 9.45 billion and is forecast to reach US$ 14.9 billion by 2033, registering a healthy CAGR of 6.7% during the forecast period. Power MOSFETs are essential semiconductor devices that enable efficient switching and…
More Releases for Epilepsy
Gavin Hogarth Shares a Personal and Informative Perspective on Living With Epile …
Image: https://www.globalnewslines.com/uploads/2026/01/1769444744.jpg
Liverpool, England - Author Gavin Hogarth offers a candid and educational look into life with epilepsy in his book, Epilepsy, What Is It? [https://www.amazon.com/Epilepsy-What-Gavin-Hogarth/dp/1836152698/ref] Drawing from his own lived experience, Hogarth shares how the condition has shaped, challenged, and changed his life, while providing practical insight for readers seeking to better understand epilepsy.
Written with honesty and purpose, Epilepsy, What Is It? aims to raise awareness and educate those who…
Adult mitochondrial epilepsy presentation
Mitochondrial epilepsy is a rare but severe neurological condition caused by genetic mutations that impair mitochondrial function, disrupting cellular energy production in the brain. Patients often present with seizures, developmental delays, and neurodegeneration. It is closely associated with broader mitochondrial diseases such as MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like Episodes) and Leigh syndrome.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/72869
Due to its rarity and complexity, treatment options…
Portable Epilepsy Monitoring Market in 2025
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Portable Epilepsy Monitoring Market- (By Product Type (Device Type (Chest Sensor, Watch Sensor, Monitors, Cameras, Mattresses and Pillows, and Others) and Service), By End User (Hospitals, Ambulatory Surgery Centres & Clinics, Neurology Centres, and Home Care Settings)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global…
Top 10 Epilepsy Companies Analysis (2024)
Epilepsy also known as seizure disorder, is a chronic noncommunicable disease that affects brain. Epilepsy affects people of all races, ethnic backgrounds, genders, and ages.
Get More Details with Sample PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00016405/?utm_source=OpenPR&utm_medium=10129
Major Companies operating in the Epilepsy Market are:
• Idexx Laboratories Inc.
• PBD Biotech Ltd
• Thermo Fisher Scientific Inc.
• Innovative Diagnostics SAS
• Neogen Corp
• Enfer Labs
• bioMerieux SA
• Ring Biotechnology Co Ltd
• Bionote Inc.
• Shenzhen Bioeasy Biotechnology Co Ltd.
Contact Us:
If you have any queries about this report or if…
Epilepsy Device Market: for Epilepsy Devices Poised to Exceed $1 Billion by 2031 …
The global epilepsy devices market isx experiencing a remarkable surge, poised to exceed a valuation of $1.1 billion by 2031, as revealed by the latest market analysis. According to the report released by Allied Market Research, the market was valued at $675.23 million in 2021 and is projected to grow at a CAGR of 5.1% from 2022 to 2031.
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐅𝐨𝐫 𝐒𝐚𝐦𝐩𝐥𝐞 𝐂𝐨𝐩𝐲: https://www.alliedmarketresearch.com/request-sample/A14821
Epilepsy devices play…
Epilepsy with Myoclonic - Atonic Seizures Therapeutics Market - Breaking the Sei …
Newark, New Castle, USA: The "Epilepsy with Myoclonic-Atonic Seizures Therapeutics Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Epilepsy with Myoclonic - Atonic Seizures Therapeutics Market: https://www.growthplusreports.com/report/epilepsy-with-myoclonic-atonic-seizures-therapeutics-market/8029
This…
