Press release
Atopic Keratoconjunctivitis Clinical Trials and Studies 2025: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies
DelveInsight's, "Atopic Keratoconjuctivitis Pipeline Insight 2025" report provides comprehensive insights about 10+ companies and 10+ pipeline drugs in Atopic Keratoconjuctivitis pipeline landscape. It covers the Atopic Keratoconjunctivitis pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Atopic Keratoconjunctivitis pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Discover the latest drugs and treatment options in the Atopic Keratoconjunctivitis Pipeline. Dive into DelveInsight's comprehensive report today! @ Atopic Keratoconjunctivitis Pipeline Outlook [https://www.delveinsight.com/sample-request/atopic-keratoconjuctivitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Atopic Keratoconjunctivitis Pipeline Report
* DelveInsight's Atopic Keratoconjunctivitis pipeline report depicts a robust space with 10+ active players working to develop 10+ pipeline therapies for Atopic Keratoconjunctivitis treatment.
* The leading Atopic Keratoconjunctivitis Companies such as Akari Therapeutics, Allakos , and others.
* Promising Atopic Keratoconjunctivitis Pipeline Therapies such as Dupilumab, Cyclosporine 0.010% and others.
Stay ahead with the most recent pipeline outlook for Atopic Keratoconjunctivitis. Get insights into clinical trials, emerging therapies, and leading companies with DelveInsight @ Atopic Keratoconjunctivitis Treatment Drugs [https://www.delveinsight.com/sample-request/atopic-keratoconjuctivitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Atopic Keratoconjunctivitis Emerging Drugs Profile
* rVA576: Akari Therapeutics
rVA576 is currently in Phase I/II of clinical trial study and is being developed by Akari Therapeutics for the treatment of Atopic Keratoconjuctivitis. Thye trial got initiated in February 2019 and is expected to get completed by February 2022.
The Atopic Keratoconjunctivitis Pipeline Report Provides Insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Atopic Keratoconjunctivitis with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Atopic Keratoconjunctivitis Treatment.
* Atopic Keratoconjunctivitis Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Atopic Keratoconjunctivitis Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Atopic Keratoconjunctivitis market
Explore groundbreaking therapies and clinical trials in the Atopic Keratoconjunctivitis Pipeline. Access DelveInsight's detailed report now! @ New Atopic Keratoconjunctivitis Drugs [https://www.delveinsight.com/sample-request/atopic-keratoconjuctivitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Atopic Keratoconjunctivitis Companies
Akari Therapeutics, Allakos , and others.
Atopic Keratoconjuctivitis pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Oral
* Parenteral
* Intravitreal
* Subretinal
* Topical.
* Molecule Type
Atopic Keratoconjunctivitis Products have been categorized under various Molecule types such as
* Monoclonal Antibody
* Peptides
* Polymer
* Small molecule
* Gene therapy
* Product Type
Unveil the future of Atopic Keratoconjunctivitis Treatment. Learn about new drugs, pipeline developments, and key companies with DelveInsight's expert analysis @ Atopic Keratoconjunctivitis Market Drivers and Barriers [https://www.delveinsight.com/sample-request/atopic-keratoconjuctivitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Atopic Keratoconjunctivitis Pipeline Report
* Coverage- Global
* Atopic Keratoconjunctivitis Companies- Akari Therapeutics, Allakos, and others.
* Atopic Keratoconjunctivitis Pipeline Therapies- Dupilumab, Cyclosporine 0.010% and others.
* Atopic Keratoconjunctivitis Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Atopic Keratoconjunctivitis Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Get the latest on Atopic Keratoconjunctivitis Therapies and clinical trials. Download DelveInsight's in-depth pipeline report today! @ Atopic Keratoconjunctivitis Companies, Key Products and Unmet Needs [https://www.delveinsight.com/sample-request/atopic-keratoconjuctivitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Atopic Keratoconjuctivitis: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Atopic Keratoconjuctivitis - DelveInsight's Analytical Perspective
* In-depth Commercial Assessment
* Atopic Keratoconjuctivitis Collaboration Deals
* Late Stage Products (Phase III)
* Drug Name: Company Name
* Mid Stage Products (Phase I/II)
* rVA576: Akari Therapeutics
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* AK002: Allakos
* Drug profiles in the detailed report.....
* Pre-clinical and Discovery Stage Products
* Drug Name: Company Name
* Drug profiles in the detailed report.....
* Inactive Products
* Atopic Keratoconjuctivitis Key Companies
* Atopic Keratoconjuctivitis Key Products
* Atopic Keratoconjuctivitis- Unmet Needs
* Atopic Keratoconjuctivitis- Market Drivers and Barriers
* Atopic Keratoconjuctivitis- Future Perspectives and Conclusion
* Atopic Keratoconjuctivitis Analyst Views
* Atopic Keratoconjuctivitis Key Companies
* 28. Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=atopic-keratoconjunctivitis-clinical-trials-and-studies-2025-ema-pdma-fda-approvals-mechanism-of-action-roa-nda-ind-and-companies]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Atopic Keratoconjunctivitis Clinical Trials and Studies 2025: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies here
News-ID: 3966143 • Views: …
More Releases from ABNewswire
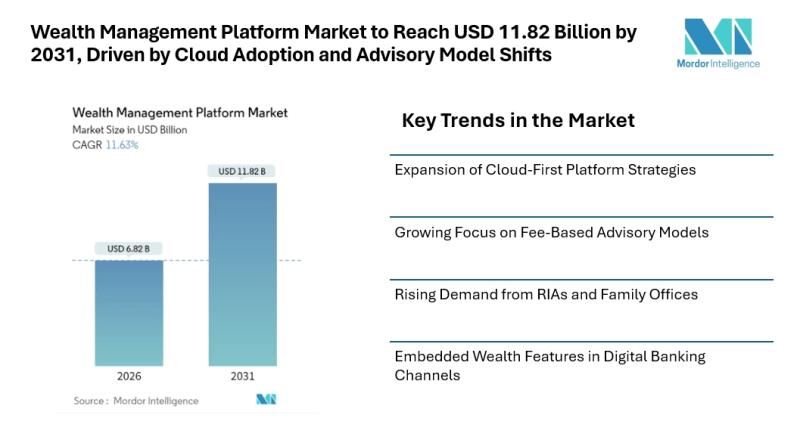
Wealth Management Platform Market to Reach USD 11.82 Billion by 2031, Driven by …
Mordor Intelligence has published a new report on the wealth management platform market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Wealth Management Platform Market Overview
The wealth management platform market continues to gain steady attention as financial institutions modernize advisory operations and respond to changing investor expectations. According to Mordor Intelligence, the wealth management platform market size [https://www.mordorintelligence.com/industry-reports/wealth-management-platform-market?utm_source=abnewswire] stood at USD 6.82 billion in 2026 and is projected…

Why UK Taxpayers Are Choosing the Best Self Assessment Software in 2026
As HMRC continues to support online filing, self assessment software has become an essential tool rather than an optional one. The best platforms help users stay organised throughout the year, not just at deadline time. Pie's approach reflects this shift, focusing on simplicity, trust and transparency, while reinforcing its core message: "It's your money. Claim it."
LONDON, United Kingdom - As self assessment deadlines approach and digital filing becomes the default,…

Beycome Secures $2.5 Million Seed Funding Round to Scale Digital Real Estate Pla …
Image: https://www.abnewswire.com/upload/2026/02/01902a4178e53eaeed8cf0351beeed89.jpg
Beycome [https://www.beycome.com/], a tech-first, direct-to-consumer real estate platform, announced today the closing of a $2.5 million seed funding round. InsurTech Fund led the oversubscribed round with participation from Pivot Ventures, Florida Opportunity Fund, RedShift Capital, Neer Venture Capital, Kima Ventures, Ignite Venture, and Founders Future, alongside several strategic angel investors.
Founded in 2020, Beycome provides a digital ecosystem that allows homeowners and buyers to conduct transactions without traditional percentage-based commissions.…

Montgomery Roofing - Lorena Roofers Enhances Roofing Maintenance Options for Hom …
Montgomery Roofing - Lorena Roofers continues to support homeowners and businesses in Lorena and nearby areas with dependable, locally focused roofing care. With an emphasis on consistent service, clear communication, and practical solutions, Montgomery Roofing - Lorena Roofers remains dedicated to protecting properties and meeting the ongoing needs of the communities it serves.
Montgomery Roofing Lorena Roofers continues to strengthen its local presence by improving access to dependable roofing maintenance [https://roofstexas.com/lorena-roofers/#:~:text=bitumen%0A%E2%80%93%20EPDM-,Roofing%20Maintenance,-Services]…
More Releases for Atopic
Rising Prevalence Of Atopic Dermatitis Is A Key Catalyst For The Growth Of The A …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Atopic Dermatitis Market Size Valuation Forecast: What Will the Market Be Worth by 2025?
The scope of the atopic dermatitis market has experienced substantial expansion recently, projected to increase from $8.61 billion in 2024 to reach $9.88 billion in 2025, demonstrating a compound annual growth rate (CAGR) of 14.7%;…
Atopic Dermatitis Market Trends That Will Shape the Next Decade: Insights from P …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Atopic Dermatitis Market Size By 2025?
The market size for atopic dermatitis has seen significant expansion in the past few years. This expansion is anticipated to continue, with the market size increasing from $8.61 billion in 2024 to $9.96 billion in 2025, exhibiting a compound annual growth rate (CAGR) of…
Atopic Dermatitis Pipeline Outlook Report 2024
DelveInsight's, "Atopic Dermatitis Pipeline Insight 2024" report provides comprehensive insights about 100+ companies and 110+ pipeline drugs in the Atopic Dermatitis pipeline landscape. It covers the pipeline drug profiles, including Atopic Dermatitis clinical trials and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Key Takeaways from the Atopic Dermatitis Pipeline…
Advanced Therapy Revolutionizes Atopic Dermatitis Market Prospects
The atopic dermatitis market size is expected to be valued at USD 15.42 Billion in 2024 and reach USD 30.5 Billion by 2029, growing at a CAGR of 14.5 % from 2024 to 2029.
Atopic dermatitis, known as eczema, is a prevalent skin condition marked by red, inflamed, and itchy skin. It is a chronic condition commonly seen in children but can also impact adults. Atopic dermatitis is thought to result…
Atopic Dermatitis - Drug Pipeline Landscape, 2023
Atopic dermatitis (eczema) is a condition that causes dry, itchy and inflamed skin. It's characterized by inflamed skin that may crak and release a clear fluid when scratched. Eczema damages the skin barrier function that makes skin more sensitive and more prone to infection and dryness. Atopic dermatitis is caused by a combination of immune system activation, genetics, environmental triggers and stress. A weak skin barrier function might also trigger…
Atopic Dermatitis - Drug Pipeline Landscape, 2022
Atopic dermatitis (eczema) is a condition that causes dry, itchy and inflamed skin. It's characterized by inflamed skin that may cr*ck and release a clear fluid when scratched. Eczema damages the skin barrier function that makes skin more sensitive and more prone to infection and dryness.
Atopic dermatitis is caused by a combination of immune system activation, genetics, environmental triggers and stress. A weak skin barrier function might also trigger an…
