Press release
Fabry Disease Treatment Market Emerging Opportunities with Focus on Awareness Campaigns and Regulatory Support for Rare Diseases
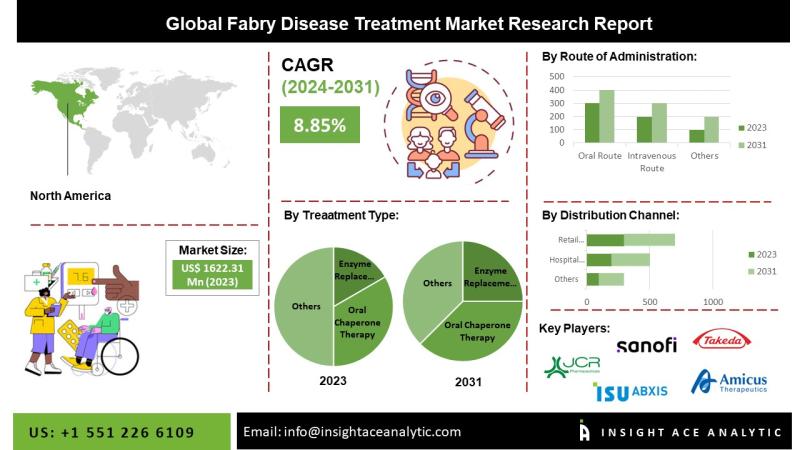
"Fabry Disease Treatment Market" in terms of revenue was estimated to be worth $1,622.31 Mn in 2023 and is poised to reach $3,150.75 Mn by 2031, growing at a CAGR of 8.21% from 2024 to 2031 according to a new report by InsightAce Analytic.Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/1451
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the Fabry disease treatment market are:
• Raise your level of awareness
• Advances in research and development
• Designated orphan drug
The following are the primary obstacles to the fabry disease treatment market's expansion:
• Limited number of patients
• High cost of treatment
• Difficulty in diagnosis
Future expansion opportunities for the global Fabry disease treatment market include:
• Advances in research and development
• Pipeline development
• International expansion
Market Analysis:
The Fabry disease treatment market encompasses a range of therapeutic strategies designed to address both the symptoms and root causes of the condition. The primary standard of care is enzyme replacement therapy (ERT), which involves the intravenous administration of a recombinant form of alpha-galactosidase A-an enzyme that is deficient or absent in individuals with Fabry disease. Additionally, chaperone therapy utilizes small molecules to stabilize and enhance the function of the mutated enzyme, thereby improving its biological activity. Another emerging approach, substrate reduction therapy, focuses on decreasing the accumulation of globotriaosylceramide (Gb3), a lipid that builds up due to the enzyme deficiency. Continuous advancements in research and ongoing clinical trials are vital to evaluating the safety, efficacy, and long-term benefits of novel therapeutic interventions for Fabry disease.
List of Prominent Players in the Fabry Disease Treatment Market:
• Sanofi Genzyme
• Share
• Amicus therapeutics
• Protalix biotherapeutics
• Idorsia pharmaceuticals
• Migal Galilee
• Greenovation biotech gmbh
• Chiesi group
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2025-04
Recent Developments:
• In May 2023, Europe granted Chiesi Farmaceutici and Protalix BioTherapeutics marketing authorization for PRX-102 (pegungalsidase alfa) for the treatment of Fabry disease in Europe. This approval will help expand treatment options for patients with Fabry disease in the region.
• In September 2022, the FDA granted Orphan Drug Designation (ODD) to AL01211 for the treatment of Fabry disease, developed by AceLink Therapeutics. This particular treatment, a glucosylceramide synthase inhibitor (GCS inhibitor), is unique because it is an oral medication that fills a significant need compared to other treatments.
• In August 2018, PerkinElmer received approval from the U.S. Food and Drug Administration (FDA) to sell the NeoLSD MSMS kit commercially. This innovative tool can detect approximately six lysosomal storage disorders in newborns, including Fabry disease, and can be easily diagnosed using a blood sample.
Fabry Disease Treatment Market Dynamics
Market Drivers: Increasing Awareness Among Healthcare Professionals
The rising awareness of Fabry disease among healthcare providers, patients, and the general public is expected to drive demand for effective treatment options. Ongoing research efforts aimed at deepening the understanding of Fabry disease and advancing therapeutic innovation are projected to support market expansion. Developments in enzyme replacement therapy, gene therapy, and other novel treatment modalities are broadening the therapeutic landscape. Additionally, given Fabry disease's classification as a rare or orphan condition, regulatory agencies offer various incentives-such as market exclusivity, tax incentives, and regulatory fee waivers-to encourage pharmaceutical companies to pursue drug development in this area. Strategic collaborations between pharmaceutical companies, academic institutions, and research organizations are also playing a crucial role by pooling expertise, funding, and resources to accelerate the development and commercialization of new therapies.
Challenges: Limited Patient Population
As a rare genetic disorder, Fabry disease affects a relatively small patient population, posing challenges for pharmaceutical companies in justifying the substantial investment required for research, development, and commercialization. The high costs associated with developing and manufacturing therapies for rare diseases often translate into elevated treatment prices, which may hinder patient access and reimbursement-particularly in cost-sensitive healthcare systems. Furthermore, the complex and often nonspecific symptomatology of Fabry disease can lead to diagnostic delays or misdiagnoses, impeding timely treatment and negatively impacting market growth. Navigating the regulatory landscape also remains a significant hurdle, as obtaining orphan drug designation, fulfilling clinical trial requirements, and achieving regulatory approval can be both time-consuming and resource-intensive. Additionally, as more therapies enter the market, heightened competition and potential product overlap may lead to market saturation, affecting pricing strategies and limiting individual product market share.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/1451
North America Expected to Exhibit the Highest CAGR During the Forecast Period
North America is projected to demonstrate the highest compound annual growth rate (CAGR) in the Fabry disease treatment market over the forecast period. This growth is supported by the region's advanced healthcare infrastructure, a robust regulatory environment conducive to orphan drug development, and a relatively higher reported prevalence of rare diseases. Enzyme replacement therapy (ERT) remains a cornerstone of treatment in this market, with several ERT products already approved and commercially available in North America. These therapies are specifically designed to address the underlying cause of Fabry disease by supplementing the deficient alpha-galactosidase A enzyme.
In addition, regulatory agencies across the region, including the U.S. Food and Drug Administration (FDA), frequently grant orphan drug status to therapies targeting rare conditions such as Fabry disease. This designation offers benefits such as market exclusivity and financial incentives, encouraging pharmaceutical firms to invest in novel treatment development. North America also serves as a hub for clinical trials focused on rare diseases, providing patients with early access to cutting-edge therapies through trial participation.
Moreover, patient advocacy organizations within the region significantly contribute to market growth by promoting disease awareness, supporting affected individuals and families, and enhancing treatment accessibility. These groups often collaborate with healthcare providers, researchers, and biopharmaceutical companies to advance knowledge and accelerate innovation in Fabry disease.
Segmentation of Fabry Disease Treatment Market-
By Type Of Treatment-
• Enzyme replacement therapy (ERT)
• Oral Chaperone Therapy
• Other Treatments
By Route of Administration
• Oral Route
• Intravenous Route
By Distribution Channel
• Hospital Pharmacies
• Retail Pharmacies
• Online Pharmacies
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Fabry Disease Treatment Market Emerging Opportunities with Focus on Awareness Campaigns and Regulatory Support for Rare Diseases here
News-ID: 3955594 • Views: …
More Releases from Insightace Analytic Pvt Ltd.
Automotive Lead Acid Battery Market Strategic Growth Drivers and Outlook 2026 to …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Lead Acid Battery Market Size, Share & Trends Analysis Report By Product (SLI and Micro-Hybrid Batteries), Type (Flooded, Enhanced Flooded, and VRLA), Customer Segment (OEM and Aftermarket), End User (Passenger Car, Light Commercial Vehicles, Heavy Commercial Vehicles, Two-Wheeler, and Three-Wheeler), and Application (Hybrid Vehicles, Electric Vehicles, Light Motor Vehicles, and Heavy Motor Vehicles)- Market…
Automotive Interior Market Investment Opportunities and Forecast 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Automotive Interior Market- (By Component Type (Center Stack, Head-up Display, Instrument Cluster, Rear Sear Entertainment, Dome Module, Headliner, Seat, Interior Lighting Door Panel, Center Console, Adhesives & Tapes, Upholstery, Others), By Material (Leather, Fabric, Vinyl, Wood, Glass Fiber Composite, Carbon Fiber Composite, Metal), By Level of Autonomy (Semi-Autonomous, Autonomous, Non-Autonomous),By Electric Vehicle (Battery Electric Vehicle…
Artificial General Intelligence Market Future Landscape and Industry Evolution 2 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Artificial General Intelligence (AGI) Market - (By Type of Offering (Hardware, Software and Service), Type of Technology (Machine Learning, Deep Learning, Natural Language Processing and Robotics), Mode of Deployment (Cloud-based, On Premise and Web-based), Type of AI (Weak AI, Strong AI and Superintelligence), Type of Processing (Image, Text and Voice Processing), Company Size (SMEs and…
Allogenic Cell Therapies Market Revenue Trends and Growth Potential 2026 to 2035
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Allogenic Cell Therapies Market- by Cell Type(Cardiosphere-Derived Cells (CDCs), Fibroblasts, T-cells, Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs) and Others),Tissue Source(Skin, Blood, PBC, BM and Others), Indication (Acute graft-versus-host disease (GVHD), Chronic Ulcers and Diabetic Foot Ulcers, Osteoarthritis, Crohn's Disease, Cardiovascular Disease, Solid Tumors/Cancers and Others (Alzheimer's Disease, etc.)), Trends, Industry Competition Analysis, Revenue…
More Releases for Fabry
Key Factor Supporting Global Fabry Disease Treatment Market Development in 2025: …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Fabry Disease Treatment Market Size By 2025?
The valuation of the Fabry disease treatment market has experienced robust expansion recently, projected to advance from $2.09 billion in 2024 up to $2.27 billion by 2025, reflecting an 8.8% compound annual growth rate during this timeframe. This…
U.S. Fabry Disease Market Size Report 2034
On April 28, 2025, Exactitude Consultancy., Ltd. released a research report titled "U.S. Fabry Disease Market". This report covers the global U.S. Fabry Disease market sales, sales volume, price, market share, ranking of major companies, etc., and provides a detailed analysis by region, country, product type, and application. It also forecasts the market size of automotive kick sensors based on market patterns from 2020 to 2034 and future market trends.…
Top Factor Driving Fabry Disease Treatment Market Growth in 2025: Impact of Incr …
How Are the key drivers contributing to the expansion of the fabry disease treatment market?
The rising prevalence of renal diseases is expected to drive the growth of the Fabry disease treatment market. Renal diseases are becoming more prevalent due to genetic factors, lifestyle choices, and environmental influences. Fabry disease, which causes kidney dysfunction, is increasing and requires timely intervention. According to the Australian Bureau of Statistics, kidney disease affected 246,200…
Fabry Disease Market Trends Analysis 2030
Fabry disease is a rare X-linked lysosomal storage disorder. This patient has a deficiency in the enzyme alpha galactosidase, which progresses to organ failure. The development of fabry illnesses is mostly caused by abnormal accumulation of a certain fatty substance known as globotriaosylceramide. This aberrant buildup can be detected in the skin, eyes, heart, kidney, brain, gastrointestinal system, and central nervous system, among other body parts.
Galactosidase Alpha (GLA) is a…
Fabry Disease - Pipeline Review, H1 2017
ReportsWorldwide has announced the addition of a new report title Fabry Disease - Pipeline Review, H1 2017 to its growing collection of premium market research reports.
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Fabry Disease - Pipeline Review, H1 2017, provides an overview of the Fabry Disease (Genetic Disorders) pipeline landscape.
Fabry disease is an inherited disorder. Fabry disease results from abnormal deposits of a particular fatty substance (called…
Fabry Disease Market Intelligence Report Offers Growth Prospects
Fabry diseaseis also known as Anderson-Fabry disease and alpha-galactosidase A deficiency. It is a rare genetic disorder of lipid metabolism resulting from the deficient activity of the alpha-galactosidase A (a-Gal A) enzyme. The deficiency of the enzyme is caused by the alterations in the genes that instructs the cells to make alpha-galactosidase A (a-Gal A) enzyme. Fabry disease is known to cause variety of systemic symptoms and complications, one of…