Press release
The Evolving Landscape of the Medical Device Regulatory Affairs Market: Trends, Challenges, and Opportunities
Market OverviewThe global medical device regulatory affairs market is experiencing rapid growth, driven by the increasing complexity of medical technologies, stringent compliance requirements, and a rising demand for efficient regulatory services. Valued at $7.0 billion in 2021, the market is projected to reach $12.2 billion by 2031, growing at a CAGR of 5.8% from 2022 to 2031.
Get a Sample Copy of this Report: https://www.alliedmarketresearch.com/request-sample/A16307
Why Regulatory Affairs Matter
Regulatory affairs professionals serve as a vital link between medical device manufacturers and regulatory authorities such as:
• FDA (U.S.) - Ensures safety and efficacy of devices.
• MHRA (UK) & EMA (EU) - Regulate medical products in Europe.
• CDSCO (India) & TGA (Australia) - Monitor compliance in emerging markets.
With medical devices incorporating cutting-edge innovations like AI-driven diagnostics, robotic surgery, and wearable health tech, regulatory expertise has become indispensable.
Key Market Drivers
✔ Increasing Use of Advanced Medical Devices - Digital health solutions and AI-powered technologies require regulatory approvals.
✔ Rising Chronic Disease Burden - More cardiovascular, cancer, and diabetes cases demand compliant medical interventions.
✔ Aging Population - The rising number of elderly patients boosts demand for regulated devices.
✔ Technological Innovations - Wearables, mobile health apps, and smart implants drive the need for compliance frameworks.
✔ Outsourcing of Regulatory Services - Many companies opt for third-party regulatory firms to streamline approvals and reduce costs.
Market Segmentation & Growth Trends
By Services
• Regulatory Consulting & Strategy - High demand for expert guidance.
• Regulatory Writing & Documentation - Crucial for compliance submissions.
• Legal Representation - Grew at 6.1% CAGR, ensuring smooth approvals.
• Product Registration & Clinical Trials - Fastest-growing at 6.8% CAGR.
By Service Provider
• Outsourcing - Leads the market with a 6.0% CAGR due to cost efficiency.
• In-House Teams - Preferred by large corporations, growing at 5.5% CAGR.
By Device Type
• Therapeutic Devices - Largest segment, growing at 5.6% CAGR.
• Diagnostic Devices - Fastest-growing at 6.2% CAGR, driven by AI-powered solutions.
By Indication
• Musculoskeletal Disorders - Highest revenue contributor (4.6% CAGR).
• Cardiovascular Diseases - Fastest-growing segment (6.5% CAGR).
Regional Market Insights
• North America - Leading due to strong regulatory frameworks and R&D investments.
• Europe - Strict EU compliance laws fuel demand for regulatory services.
• Asia-Pacific - Fastest-growing market with increasing clinical trials.
• LAMEA - Emerging regulatory market with improving healthcare infrastructure.
Challenges in the Regulatory Affairs Market
⚠ High Costs of Compliance - Regulatory approvals require substantial financial investment. ⚠ Cybersecurity Threats - Digital medical devices face rising cyber risks. ⚠ Diverse Approval Processes - Regulatory variations across countries delay market entry.
Future Outlook & Emerging Opportunities
🔹 AI & Digital Health Regulations - New policies for AI-driven diagnostics and telemedicine. 🔹 Global Standards Harmonization - Efforts to unify compliance across regions. 🔹 Expansion in Emerging Markets - Increasing regulatory focus in Asia and Africa.
Key Industry Players
Some of the leading companies shaping the medical device regulatory affairs market include:
• AmerisourceBergen
• Charles River Laboratories
• ICON PLC
• IQVIA
• Parexel
• Pepgra
Final Thoughts
The medical device regulatory affairs market is evolving at a fast pace, driven by technological advancements, rising healthcare needs, and stricter regulations. Companies investing in efficient compliance strategies, outsourcing, and regulatory expertise will gain a competitive edge in this rapidly expanding industry.
Would you like insights on a specific segment or region? Let me know how I can refine this further! 🚀
Enquire Before Buying: https://www.alliedmarketresearch.com/purchase-enquiry/A16307
About Us
Allied Market Research (AMR) is a full-service market research and business-consulting wing of Allied Analytics LLP based in Portland, Oregon. Allied Market Research provides global enterprises as well as medium and small businesses with unmatched quality of "Market Research Reports" and "Business Intelligence Solutions." AMR has a targeted view to provide business insights and consulting to assist its clients to make strategic business decisions and achieve sustainable growth in their respective market domain.
Pawan Kumar, the CEO of Allied Market Research, is leading the organization toward providing high-quality data and insights. We are in professional corporate relations with various companies and this helps us in digging out market data that helps us generate accurate research data tables and confirms utmost accuracy in our market forecasting. Each and every data presented in the reports published by us is extracted through primary interviews with top officials from leading companies of domain concerned. Our secondary data procurement methodology includes deep online and offline research and discussion with knowledgeable professionals and analysts in the industry.
Contact:
David Correa
5933 NE Win Sivers Drive
#205, Portland, OR 97220
United States
Toll-Free: 1-800-792-5285
UK: +44-845-528-1300
Hong Kong: +852-301-84916
India (Pune): +91-20-66346060
Fax: +1-855-550-5975
help@alliedmarketresearch.com
Web: https://www.alliedmarketresearch.com
Follow Us on: LinkedIn Twitter
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release The Evolving Landscape of the Medical Device Regulatory Affairs Market: Trends, Challenges, and Opportunities here
News-ID: 3951458 • Views: …
More Releases from Allied Market Research
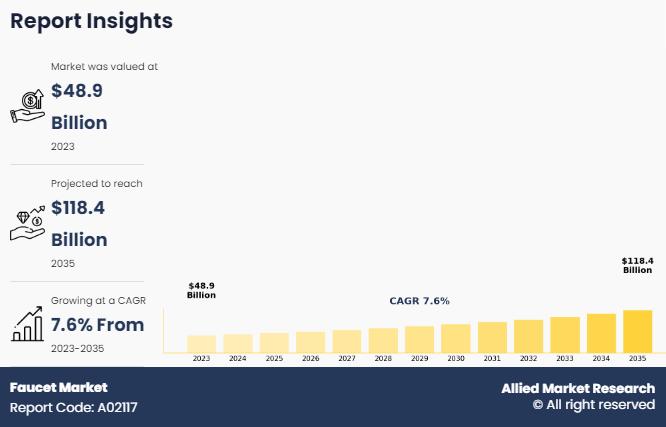
Faucet Market Forecast 2035: Reaching USD 118.4 billion by 2035
According to a new report published by Allied Market Research, titled, "Faucet Market," The faucet market size was valued at $48.9 billion in 2023, and is estimated to reach $118.4 billion by 2035, growing at a CAGR of 7.6% from 2023 to 2035.
Request The Sample PDF Of This Report: https://www.alliedmarketresearch.com/request-sample/2448
Faucet is a plumbing fixture used to control the flow of water in various settings such as kitchens,…

Vinyl Wallpaper Market Size Forecasted to Grow at 3.3% CAGR, Reaching USD 1.3 bi …
The Vinyl Wallpaper Market Size was valued at $943.30 million in 2021, and is estimated to reach $1.3 billion by 2031, growing at a CAGR of 3.3% from 2022 to 2031.
Request The Sample PDF Of This Report: https://www.alliedmarketresearch.com/request-sample/16970
Vinyl wallpaper consists of a carrier layer (recycled paper or non-woven wallpaper base) and a decorative layer made of polyvinyl chloride. A synthetic foam layer provides three-dimensional structures to…
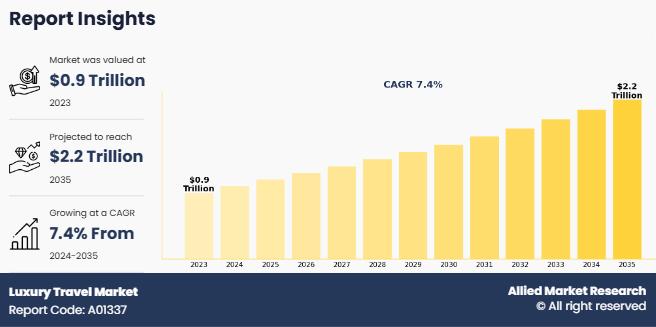
Luxury Travel Market Set to Achieve a Valuation of US$ 2149.7 billion, Riding on …
According to a new report published by Allied Market Research, titled, "Luxury Travel Market," The luxury travel market size was valued at $890.8 billion in 2023, and is estimated to reach $2149.7 billion by 2035, growing at a CAGR of 7.4% from 2024 to 2035.
Get Sample PDF Of This Report: https://www.alliedmarketresearch.com/request-sample/1662
Luxury travel refers to travel experiences that offer exceptional comfort, exclusivity, and personalized services, typically catering to…

Men Personal Care Market to Grow at a CAGR of 8.6% and will Reach USD 276.9 bill …
According to a new report published by Allied Market Research, titled, "Men Personal Care Market by Type, Age Group, Price Point, and Distribution Channel: Global Opportunity Analysis and Industry Forecast, 2021-2030," the men personal care market size is expected to reach $276.9 billion by 2030 at a CAGR of 8.6% from 2021 to 2030.
Request The Sample PDF of This Report: @ https://www.alliedmarketresearch.com/request-sample/1701
Men personal care products are non-medicinal…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
