Press release
Acute Lymphocytic Leukemia Clinical Trials and Studies 2025: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies
DelveInsight's, "Acute Lymphocytic Leukemia Pipeline Insight 2025" report provides comprehensive insights about 125+ companies and 130+ pipeline drugs in Acute Lymphocytic Leukemia pipeline landscape. It covers the Acute Lymphocytic Leukemia pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Acute Lymphocytic Leukemia pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our latest breakthroughs in Acute Lymphocytic Leukemia Research. Learn more about our innovative pipeline today! @ Acute Lymphocytic Leukemia Pipeline Outlook [https://www.delveinsight.com/sample-request/acute-lymphocytic-leukemia-all-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Acute Lymphocytic Leukemia Pipeline Report
* In March 2025, Janssen Research & Development, LLC conducted a study is to evaluate the efficacy of daratumumab in addition to standard chemotherapy in pediatric participants with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL)/lymphoblastic lymphoma (LL) and T-cell ALL/LL as measured by the complete response (CR) rate.
* In March 2025, Pfizer organized a phase 2 study is designed to evaluate the superiority of InO monotherapy vs ALLR3 after 1 cycle of induction treatment in paediatric participants (between 1 and
* In March 2025, Amgen announced a phase 2 Study to Evaluate Efficacy, Safety, and Pharmacokinetics (PK) of Blinatumomab in Chinese Pediatric Subjects With Relapsed or Refractory B Precursor Acute Lymphoblastic Leukemia (R/R B-ALL).
* In March 2025, AbbVie organized an extension study is to provide venetoclax and obtain long-term safety data for subjects who continue to tolerate and derive benefit from receiving venetoclax in ongoing studies.
* DelveInsight's Acute Lymphocytic Leukemia pipeline report depicts a robust space with 125+ active players working to develop 130+ pipeline therapies for Acute Lymphocytic Leukemia treatment.
* The leading Acute Lymphocytic Leukemia Companies such as Orca Biosystems, Inc, Jazz Pharmaceuticals, Shenzhen TargetRx, Inc, Cellectis, ADC Therapeutics S.A., Kunming Hope of Health Hospital, Beam Therapeutics Inc., Shenzhen BinDeBio Ltd., Kite, A Gilead Company, Sumitomo Pharma America, Inc., In8bio Inc., Fate Therapeutics, Sichuan Baili Pharmaceutical Co., Ltd., Janssen Research & Development, LLC, Medolution Ltd., Kymera Therapeutics, Inc., Kite, A Gilead Company | Gilead Sciences, Newave Pharmaceutical Inc, Servier, Meryx, Inc., Armaceutica, Inc., Hangzhou Qihan Biotech Co., Ltd., Nanjing Bioheng Biotech, Sanofi, Syndax Pharmaceuticals, Vincerx Pharma, Inc. and others.
* Promising Acute Lymphocytic Leukemia Pipeline Therapies such as Omitted Doxorubicin, Blinatumomab, Dexamethasone, Vincrisitne, Recombinant Asparaginase, Inotuzumab Ozogamicin and others.
Stay informed about the cutting-edge advancements in Acute Lymphocytic Leukemia treatments. Download for updates and be a part of the revolution in care @ Acute Lymphocytic Leukemia Clinical Trials Assessment [https://www.delveinsight.com/sample-request/acute-lymphocytic-leukemia-all-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Acute Lymphocytic Leukemia Emerging Drugs Profile
* Orca-T: Orca Biosystems, Inc.
Orca-T is an investigational high-precision cell therapy designed to replace a patient's cancerous blood and immune system with a healthy one while dramatically lowering their risk of developing GvHD and other potentially life-threatening side effects. In the Phase Ib/II study, when measured against a concurrent, nonrandomized single-center comparator for allogeneic transplant patients, Orca-T demonstrated preliminary evidence of significantly higher GvHD-free, relapse-free survival rates after 1 year, improved relapse-free survival rates and lower rates of chronic GvHD. Currently, the drug is in Phase III stage of its development for the treatment of ALL.
* TGRX-814: Shenzhen TargetRx, Inc.
TGRX-814 is a highly selective inhibitor of the BCL2 (over BCL-XL) for the treatment of CLL with or without the del(17p)/TP53 mutation, NHL, SLL, DLBL, MM, etc. Notably, TGRX-814 has an excellent selectivity profile for BCL-XL. TGRX-814 is modified and optimized through classical bioisosterism and molecule simulation technologies. In-vitro and in-vivo assays have shown that TGRX-814 improves oral metabolism, increases in vivo exposure, and reduces compound clearance while maintaining in vitro and in vivo bioactivity. Moreover, TGRX-814 obtained over 2-fold increase in bioavailability compared to the marketed drug Venetoclax, resulting in a significant increase in efficacy. Currently, the drug is in Phase I/II stage of its development for the treatment of ALL.
* UCART22: Cellectis
UCART22 is one of Cellectis' wholly owned, allogeneic, off-the-shelf gene-edited T-cell product candidates designed for the treatment of relapsed and refractory B-cell acute lymphoblastic leukemia (R/R B-ALL). Like CD19, CD22 is a cell surface antigen expressed from the pre-B-cell stage of development through mature B-cells. CD22 expression occurs in more than 90% of patients with B-ALL. It is currently in phase I stage of development. Currently, the drug is in Phase I stage of its development for the treatment of ALL.
The Acute Lymphocytic Leukemia Pipeline Report Provides Insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Acute Lymphocytic Leukemia with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Acute Lymphocytic Leukemia Treatment.
* Acute Lymphocytic Leukemia Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Acute Lymphocytic Leukemia Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Acute Lymphocytic Leukemia market
Learn more about Acute Lymphocytic Leukemia Drugs opportunities in our groundbreaking Colo Acute Lymphocytic Leukemia Research and development projects @ Acute Lymphocytic Leukemia Unmet Needs [https://www.delveinsight.com/sample-request/acute-lymphocytic-leukemia-all-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Acute Lymphocytic Leukemia Companies
Orca Biosystems, Inc, Jazz Pharmaceuticals, Shenzhen TargetRx, Inc, Cellectis, ADC Therapeutics S.A., Kunming Hope of Health Hospital, Beam Therapeutics Inc., Shenzhen BinDeBio Ltd., Kite, A Gilead Company, Sumitomo Pharma America, Inc., In8bio Inc., Fate Therapeutics, Sichuan Baili Pharmaceutical Co., Ltd., Janssen Research & Development, LLC, Medolution Ltd., Kymera Therapeutics, Inc., Kite, A Gilead Company | Gilead Sciences, Newave Pharmaceutical Inc, Servier, Meryx, Inc., Armaceutica, Inc., Hangzhou Qihan Biotech Co., Ltd., Nanjing Bioheng Biotech, Sanofi, Syndax Pharmaceuticals, Vincerx Pharma, Inc. and others.
Acute Lymphocytic Leukemia (ALL) pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Acute Lymphocytic Leukemia Products have been categorized under various Molecule types such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene therapy
Discover the latest advancements in Acute Lymphocytic Leukemia Treatment by visiting our website. Stay informed about how we're transforming the future of disease @ Acute Lymphocytic Leukemia Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/acute-lymphocytic-leukemia-all-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Acute Lymphocytic Leukemia Pipeline Report
* Coverage- Global
* Acute Lymphocytic Leukemia Companies- Orca Biosystems, Inc, Jazz Pharmaceuticals, Shenzhen TargetRx, Inc, Cellectis, ADC Therapeutics S.A., Kunming Hope of Health Hospital, Beam Therapeutics Inc., Shenzhen BinDeBio Ltd., Kite, A Gilead Company, Sumitomo Pharma America, Inc., In8bio Inc., Fate Therapeutics, Sichuan Baili Pharmaceutical Co., Ltd., Janssen Research & Development, LLC, Medolution Ltd., Kymera Therapeutics, Inc., Kite, A Gilead Company | Gilead Sciences, Newave Pharmaceutical Inc, Servier, Meryx, Inc., Armaceutica, Inc., Hangzhou Qihan Biotech Co., Ltd., Nanjing Bioheng Biotech, Sanofi, Syndax Pharmaceuticals, Vincerx Pharma, Inc. and others.
* Acute Lymphocytic Leukemia Pipeline Therapies- Omitted Doxorubicin, Blinatumomab, Dexamethasone, Vincrisitne, Recombinant Asparaginase, Inotuzumab Ozogamicin and others.
* Acute Lymphocytic Leukemia Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Acute Lymphocytic Leukemia Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
For a detailed overview of our latest research findings and future plans, read the full details of Acute Lymphocytic Leukemia Pipeline on our website @ Acute Lymphocytic Leukemia Emerging Drugs and Companies [https://www.delveinsight.com/sample-request/acute-lymphocytic-leukemia-all-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Acute-Lymphocytic-Leukemia: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Acute-Lymphocytic-Leukemia- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Orca-T: Orca Biosystems, Inc.
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* Product Name: Company Name
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I/II)
* TGRX-814: Shenzhen TargetRx, Inc.
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug Name: Company Name
* Drug profiles in the detailed report.....
* Inactive Products
* Acute-Lymphocytic-Leukemia Key Companies
* Acute-Lymphocytic-Leukemia Key Products
* Acute-Lymphocytic-Leukemia- Unmet Needs
* Acute-Lymphocytic-Leukemia- Market Drivers and Barriers
* Acute-Lymphocytic-Leukemia- Future Perspectives and Conclusion
* Acute-Lymphocytic-Leukemia Analyst Views
* Acute-Lymphocytic-Leukemia Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=acute-lymphocytic-leukemia-clinical-trials-and-studies-2025-ema-pdma-fda-approvals-mechanism-of-action-roa-nda-ind-and-companies]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/medical-marijuana-market-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Acute Lymphocytic Leukemia Clinical Trials and Studies 2025: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies here
News-ID: 3940370 • Views: …
More Releases from ABNewswire

When a Medical Mistake Becomes the Breaking Point in a Marriage
When a family expects healing but receives bad news instead, everything they planned for the future shakes at once. A medical error does not simply alter a treatment plan. It changes daily routines, responsibilities and the emotional balance that spouses lean on to stay steady. Couples often describe the first weeks after the mistake as a blur because they try to care for the injured person while also sorting through…

Top Oncology Conferences in 2026: A Ranked Guide for Industry and Health System …
A comprehensive analysis of upcoming oncology conferences taking place in 2026, and why industry and health system leaders may want to attend each one.
As oncology continues to evolve under pressure from clinical innovation, financial scrutiny, and operational complexity, the value of the right in-person forum has never been higher. For leaders navigating cancer care delivery, drug development, diagnostics, and health system strategy, conferences remain one of the few environments where…
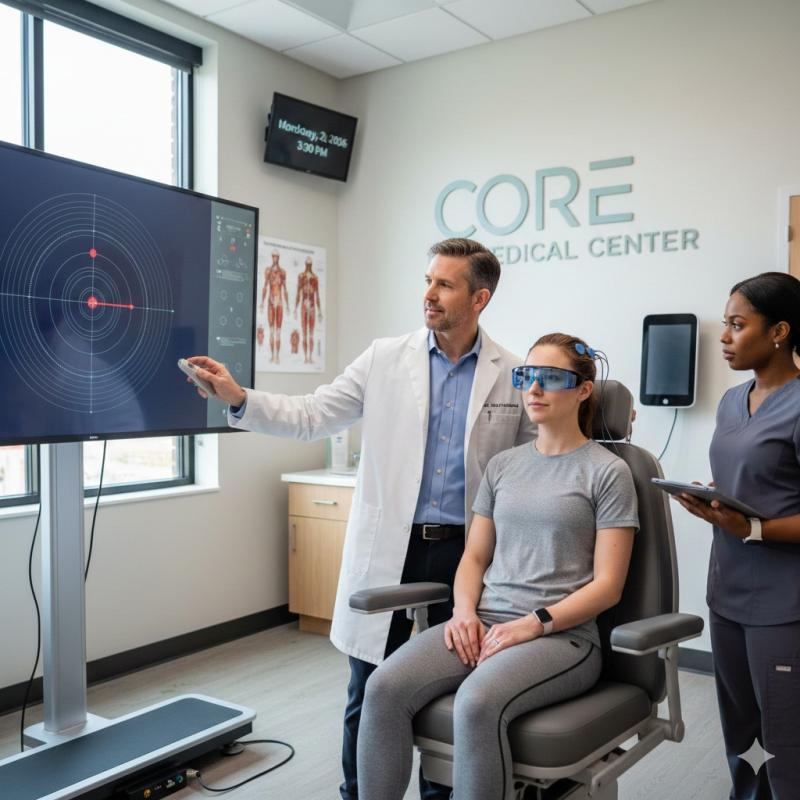
Core Medical Center Now Offering Comprehensive Concussion Treatment in Blue Spri …
Core Medical Center in Blue Springs, MO, has launched a new comprehensive concussion treatment program led by Dr. Aston Goldsworth, DC, MSN, FNP-BC. Addressing a critical gap in local post-concussion care, the practice offers a multi-modal approach combining vestibular rehab, physical therapy, and nutritional support. By integrating chiropractic and advanced nursing expertise, the clinic provides personalized recovery plans for athletes, accident victims, and seniors.
BLUE SPRINGS, MO - Core Medical Center,…

The Silent Courtyard and the Architecture of Perception
A Weston residence reframes luxury living through contemplative spatial sequencing, merging art, nature, and experiential design through Studio KHORA's architectural language.
Among the evolving discourse shaped by Top Florida architects [https://www.studiokhora.com/silent-courtyard-top-florida-architects], Studio KHORA introduces The Silent Courtyard, a residence in Weston commissioned by a Peruvian entrepreneur and developer. The project reflects a cultivated sensibility toward architecture as narrative-where entry sequences dissolve the threshold between domesticity and curated gallery experience. The house…
More Releases for Acute
Acute Lymphoblastic Leukemia Therapeutics Market Size, Acute Lymphoblastic Leuke …
Acute Lymphoblastic Leukemia Therapeutics Market Dynamics are clarified by an in-depth review of facts on current and emerging trends. To understand a resource, the paper uses Porter's five forces to examine the importance of numerous qualities such as understanding of suppliers and customers, dangers provided by various agents, competitive strength, and promising new businesses. precious. Furthermore, the study covers numerous firms' Acute Lymphoblastic Leukemia Therapeutics research data, benefit, gross margin,…
Acute Conjunctivitis Treatment Market: The Future Insights Of Global Acute Conju …
Acute Conjunctivitis Treatment Market
Acute Conjunctivitis Treatment Market, or pink eye, is an irritation or inflammation of the conjunctiva that covers the white part of the eyeball. It can be caused by allergies or a bacterial or viral infection. Acute Conjunctivitis Treatment Market can be highly communicable and can be spread easily by the contact with secretions of eye from the infected person. Conjunctivitis often resolves on its own, but…
Acute Renal Failure (ARF) (Acute Kidney Injury) Global Clinical Trials Review, H …
ReportsWorldwide has announced the addition of a new report title Acute Renal Failure (ARF) (Acute Kidney Injury) Global Clinical Trials Review, H1, 2017 to its growing collection of premium market research reports.
GlobalData's clinical trial report, “Acute Renal Failure (ARF) (Acute Kidney Injury) Global Clinical Trials Review, H1, 2017" provides an overview of Acute Renal Failure (ARF) (Acute Kidney Injury) clinical trials scenario. This report provides top line data relating to…
Acute Lymphocytic Leukemia (ALL, Acute Lymphoblastic Leukemia) Global Clinical T …
ReportsWorldwide has announced the addition of a new report title Acute Lymphocytic Leukemia (ALL, Acute Lymphoblastic Leukemia) Global Clinical Trials Review, H1, 2017 to its growing collection of premium market research reports.
GlobalData's clinical trial report, “Acute Lymphocytic Leukemia (ALL, Acute Lymphoblastic Leukemia) Global Clinical Trials Review, H1, 2017" provides an overview of Acute Lymphocytic Leukemia (ALL, Acute Lymphoblastic Leukemia) clinical trials scenario. This report provides top line data relating to…
Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia) Global Clinical Tri …
ReportsWorldwide has announced the addition of a new report title Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia) Global Clinical Trials Review, H1, 2017 to its growing collection of premium market research reports.
GlobalData's clinical trial report, “Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia) Global Clinical Trials Review, H1, 2017" provides an overview of Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia) clinical trials scenario. This report provides top line data relating to…
H2 2016 Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia) - Therapeut …
Albany, New York, January 03, 2017: A recent forecast report focusing on the Acute Myelocytic Leukemia Market has been added to the wide portfolio of Market Research Hub (MRH). It is entitled as, “Acute Myelocytic Leukemia (AML, Acute Myeloblastic Leukemia) - Pipeline Review, H2 2016” which has been equipped by in-depth market examination with inputs from the industry experts. It covers the current market scenario and also its future growth…
