Press release
Irganox: Advancing Impurity Standards for Polymers, Coatings, and Packaging to Ensure Durability, Stability, and Sustainability
Irganox impurity standards are revolutionizing the way industries analyze and maintain the integrity of antioxidants used in polymers, coatings, and packaging. By providing precise reference benchmarks for impurities, Irganox impurity standards support effective quality control, stability testing, and research efforts. These standards empower industries to ensure the highest levels of material durability, stability, and safety while paving the way for sustainable manufacturing practices.To know moe about the Irganox : https://aquigenbio.com/products/impurity-standards/irganox/
The Role of Irganox Impurity Standards in Quality Assurance
Irganox impurity standards play a critical role in defining and maintaining the functionality of antioxidants used across industrial applications. Carefully calibrated to meet rigorous analytical requirements, these standards enable researchers and manufacturers to detect, isolate, and quantify impurities in antioxidant compositions. Whether used in polymer stabilization, coatings, or packaging solutions, these standards ensure that materials perform consistently and reliably under variable environmental conditions.
To know more about the products :
https://aquigenbio.com/product/irganox-1010-d8/
https://aquigenbio.com/product/irganox-1076/
https://aquigenbio.com/product/irganox-1010/
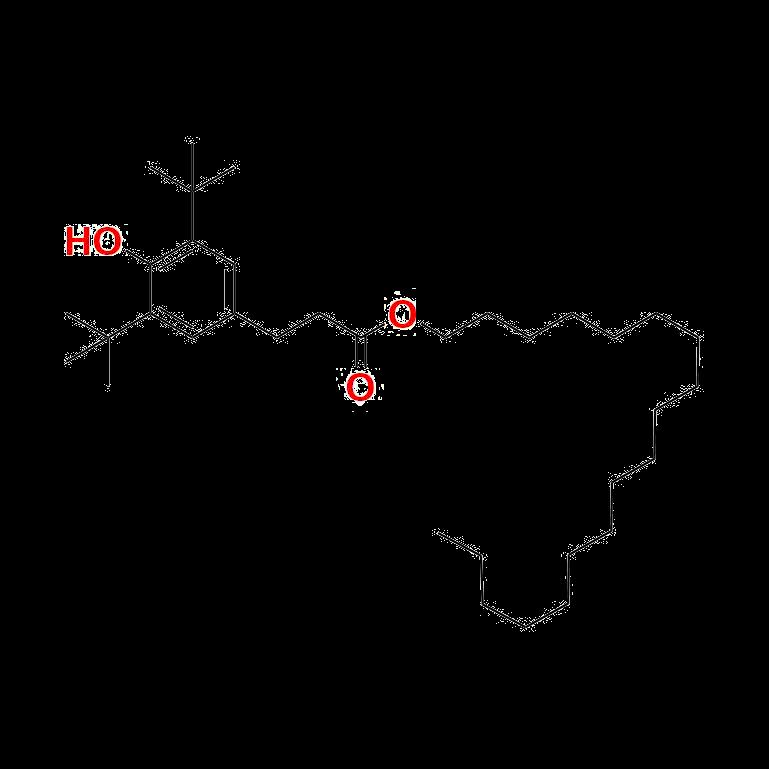
One of the most widely used antioxidants, Irganox 1010, has become a cornerstone of polymer stabilization due to its excellent thermal stability and oxidative resistance. To support precise testing of materials utilizing this compound, Irganox impurity standards offer highly refined benchmarks that ensure compliance with quality regulations. Similarly, Irganox 1076, another vital antioxidant, benefits from impurity standards tailored to meet the stringent requirements of industrial applications, ensuring the compound offers long-term protection against degradation.
In advancing analytical research, innovative solutions such as Irganox 1010-D8, a deuterated version of Irganox 1010, further enhance the precision of impurity profiling. This specialized compound is utilized in areas such as isotope labeling studies and advanced stability research. It empowers laboratories to conduct highly accurate analyses and accelerate innovation in the development of cutting-edge antioxidant solutions.
Ensuring Material Stability Through Accurate Impurity Detection
The performance of antioxidants like Irganox depends on their chemical purity and stability. Impurity standards establish necessary benchmarks for detecting unwanted contaminants that may compromise the antioxidant's efficiency or lead to material degradation. These standards are integral to industries like plastics, packaging, and coatings, where the structural integrity and longevity of materials are crucial.
By applying Irganox impurity standards during synthesis and quality control processes, manufacturers can identify degradation byproducts, process impurities, and other anomalies to ensure superior product performance. For example, in food packaging, the interaction of antioxidants with the product can be monitored and refined using accurate impurity profiling, safeguarding both consumer safety and packaging durability. Similarly, in industrial polymers and coatings, impurity standards help ensure that products retain their environmental resistance and aesthetic appeal over extended lifespans.
Supporting Regulatory Compliance and Sustainability
Global industries are increasingly governed by stringent regulatory requirements and sustainability goals. Irganox impurity standards align with these priorities by providing reliable tools to ensure materials comply with safety specifications and environmentally responsible practices. Detecting process-related impurities contributes to waste reduction by mitigating risks of product failures and recalls during or after manufacturing.
Moreover, impurity standards contribute to sustainability by refining recycling processes. For instance, in polymer recycling, impurity testing ensures that additives such as Irganox antioxidants perform optimally even after multiple recycling cycles. This approach enhances the feasibility of circular economies by reducing dependency on virgin materials and limiting environmental impact.
Key Applications of Irganox Impurity Standards
Impurity standards for Irganox antioxidants address diverse applications across global industries:
Polymers: Ensuring thermal and oxidative stability during processing and application.
Coatings: Enhancing long-term resistance to environmental factors such as UV exposure and heat.
Packaging: Safeguarding the integrity of perishable goods and maintaining material structure.
Advanced Research: Supporting analytical studies with specialized solutions such as Irganox 1010-D8 for precision testing and isotope labeling.
Whether for quality assurance, process optimization, or regulatory compliance, Irganox impurity standards provide indispensable support to industries aiming to deliver high-performance materials.
Conclusion
The importance of accurate impurity profiling in ensuring the performance and safety of antioxidants like Irganox cannot be overstated. With advanced solutions for analyzing and maintaining the purity of industry-leading antioxidants such as Irganox 1010, Irganox 1076, and Irganox 1010-D8, these impurity standards form the foundation of quality assurance and innovation in polymers, coatings, and packaging. By empowering industries with tools for precision and compliance, Irganox impurity standards strengthen the reliability and sustainability of modern materials.
Contact:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences
Aquigen Bio Sciences is India's leading provider of impurity standards, empowering global industries with reliable benchmarks for quality control and research. With extensive expertise in analytical standards, Aquigen supports industries in developing and maintaining the highest-performing materials. As the premier resource for Irganox impurity standards in India, Aquigen Bio Sciences delivers solutions tailored to meet regulatory, research, and industrial demands. By prioritizing precision, quality, and sustainability, Aquigen Bio Sciences continues to be a trusted partner in advancing material science and innovation.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Irganox: Advancing Impurity Standards for Polymers, Coatings, and Packaging to Ensure Durability, Stability, and Sustainability here
News-ID: 3920186 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for Irganox
Strategic Forecast for the Plastic Additives Industry: Market Outlook 2025-2034
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
Plastic Additives Market Size Growth Forecast: What to Expect by 2025?
In recent years, the size of the plastic additives market has seen robust growth. The market is projected to expand from a value of $59.44 billion in 2024 to $64.27 billion in 2025, indicating a compound annual growth…
Plastic Additive Market to Reach USD 71,568.1 million by 2034
The plastic additive market size is expected to reach a valuation of USD 45,595.6 million in 2024. By 2034, it is projected to grow further, reaching USD 71,568.1 million, driven by a 4.6% CAGR fueled by increased commercial viability and new manufacturing opportunities.
The plastic additive Industry is expanding due to various factors, such as technological advancements, environmental regulations, and the growing demand for eco-friendly products. Key players in the industry…
Plastic Additive Market to Reach USD 71,568.1 million by 2034
The plastic additive market size is expected to reach a valuation of USD 45,595.6 million in 2024. By 2034, it is projected to grow further, reaching USD 71,568.1 million, driven by a 4.6% CAGR fueled by increased commercial viability and new manufacturing opportunities.
The plastic additive Industry is expanding due to various factors, such as technological advancements, environmental regulations, and the growing demand for eco-friendly products. Key players in the industry…
Plastic Additive Market to Reach USD 71,568.1 million by 2034, Driven by Increas …
The plastic additive market size is expected to reach a valuation of USD 45,595.6 million in 2024. By 2034, it is projected to grow further, reaching USD 71,568.1 million, driven by a 4.6% CAGR fueled by increased commercial viability and new manufacturing opportunities.
The plastic additive Industry is expanding due to various factors, such as technological advancements, environmental regulations, and the growing demand for eco-friendly products. Key players in the industry…
Flake Copper Powder Market 2025: Industry Trends, Future Developments, and Forec …
Flake Copper Powder Market Overview
The Flake Copper Powder Market was valued at USD 12.68 billion in 2023 and is projected to grow from USD 13.41 billion in 2024 to USD 21.01 billion by 2032. The market is expected to experience a compound annual growth rate (CAGR) of 5.77% during the forecast period from 2025 to 2032.
Download Report Sample Copy: https://www.wiseguyreports.com/sample-request?id=615100
Market Dynamics
Demand Drivers
One of the primary drivers of the flake copper…
Plastic Additives Market Outlook And Industry Analysis Report 2024-2033
The Business Research Company recently released a comprehensive report on the Global Plastic Additives Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…