Press release
Drug Repurposing Market Set to Reach USD 47.8 Billion by 2034, Driven by Cost Efficiency and AI Integration |TMR
The global drug repurposing market is experiencing substantial growth, driven by the increasing demand for cost-effective drug development and advancements in artificial intelligence (AI). Drug repurposing, also known as drug repositioning, involves identifying new therapeutic uses for existing drugs, significantly reducing development time and costs. This approach has gained traction as pharmaceutical companies seek faster alternatives to traditional drug discovery, which can take over a decade and cost billions of dollars.With a market valuation of USD 28.9 billion in 2023, the industry is projected to reach USD 47.8 billion by 2034, growing at a CAGR of 4.7%. The market is witnessing significant momentum due to breakthroughs in oncology, central nervous system (CNS) disorders, and rare diseases, where unmet medical needs remain a challenge. Additionally, AI-driven analytics and big data integration are accelerating drug repurposing efforts by identifying novel drug-disease interactions with greater precision.
Access key findings and insights from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=59610
Understanding Drug Repurposing and Its Impact
A Cost-Efficient Alternative to Traditional Drug Development
The process of developing a new drug from scratch is lengthy and expensive, often requiring 10-15 years of research, testing, and regulatory approvals. In contrast, drug repurposing allows companies to leverage existing safety and efficacy data, bypassing early-stage trials and expediting regulatory approvals. This significantly reduces costs and enhances the probability of success.
Notable success stories include:
Thalidomide, initially developed as a sedative but later repurposed to treat multiple myeloma and leprosy.
Aspirin, once used for pain relief, now widely prescribed for cardiovascular disease prevention.
Sildenafil (Viagra), originally intended for hypertension, found its blockbuster application in erectile dysfunction treatment.
These examples highlight how drug repurposing can expand treatment options, improve patient outcomes, and generate new revenue streams for pharmaceutical companies.
Factors Driving the Growth of the Drug Repurposing Market
The expanding market for drug repurposing is being fueled by several key factors that are reshaping the pharmaceutical landscape.
Cost-Effective Drug Development and Reduced Failure Rates
One of the primary reasons for the growing adoption of drug repurposing is its cost efficiency. The traditional drug development process is associated with high attrition rates, with many drug candidates failing in the later stages of clinical trials. Since repurposed drugs have already been tested for safety, they are less likely to fail, making them an attractive option for pharmaceutical companies looking to optimize their research and development (R&D) investments.
AI-Driven Innovations Enhancing Drug Discovery
Artificial intelligence (AI) and machine learning algorithms are revolutionizing the way drug repurposing is conducted. AI can analyze vast amounts of clinical, genetic, and chemical data to predict how existing drugs might interact with new disease targets. Leading pharmaceutical companies are partnering with AI-driven biotech firms to accelerate drug discovery and identify new therapeutic applications for existing drugs.
For example, IBM Watson and BenevolentAI are utilizing deep learning models to analyze scientific literature and uncover hidden relationships between drugs and diseases. These AI-driven platforms can identify potential candidates for repurposing much faster than traditional methods, driving market growth.
Growing Demand for Treatments in Oncology and Neurological Disorders
The oncology sector remains the largest market for drug repurposing, as cancer treatment often requires multi-targeted therapies. Many existing cancer drugs are being repurposed for different tumor types, offering patients faster access to innovative treatments.
Similarly, neurological disorders such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis are becoming key areas of focus. Since these diseases have complex pathophysiology, repurposing existing drugs offers a viable strategy to address unmet medical needs.
Explore our report to uncover in-depth insights -
https://www.transparencymarketresearch.com/drug-repurposing-market.html
Regulatory Support and Fast-Track Approvals
Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are increasingly supporting drug repurposing initiatives by providing fast-track approval pathways. Programs like the FDA's Breakthrough Therapy Designation encourage companies to explore new applications for existing drugs, expediting their time to market.
Despite these advantages, intellectual property (IP) challenges remain a barrier to market expansion. Many repurposed drugs are off-patent, making it difficult for pharmaceutical companies to secure market exclusivity. However, companies are now leveraging data exclusivity and regulatory protections to maximize commercial potential.
Segmentation of the Drug Repurposing Market
The drug repurposing market can be segmented based on disease indication, approach type, and end-user categories:
By Disease Indication
Oncology (Largest Segment) - Repurposed cancer drugs for multiple indications.
Neurological Disorders - Alzheimer's, Parkinson's, and multiple sclerosis.
Infectious Diseases - Tuberculosis, HIV, and COVID-19 treatments.
Rare Diseases - Drug repurposing is critical due to limited treatment options.
By Approach Type
Disease-centric - Analyzing disease pathways to match drugs to new indications.
Target-centric - Identifying drug-protein interactions for new applications.
Drug-centric - Exploring how existing drugs can treat additional conditions.
By End-User
Hospitals & Clinics - Implementing repurposed drugs for expanded treatments.
Research Institutes - Conducting clinical trials on repurposed compounds.
Pharmaceutical Companies - Driving commercialization strategies for repurposed drugs.
Geographical Trends in the Drug Repurposing Market
North America
North America holds the largest market share due to high R&D investments, advanced AI applications, and strong regulatory frameworks. The U.S. FDA's support for fast-track approvals has enabled rapid commercialization of repurposed drugs.
Europe
The European market is expanding due to the EMA's focus on rare diseases and oncology. Several government-funded programs are supporting drug repurposing research, particularly in France, Germany, and the U.K.
Asia-Pacific
Asia-Pacific is emerging as a key market, with countries like China, Japan, and India investing in AI-driven drug discovery and clinical research. The region's large patient population and cost-effective trials are making it an attractive hub for pharmaceutical innovations.
Competitive Landscape and Key Players
The drug repurposing market is highly competitive, with major pharmaceutical and biotech companies investing in AI-powered repurposing strategies.
Leading Companies in Drug Repurposing
Pfizer Inc. - Focused on oncology and rare disease repurposing.
Novartis AG - Developing new indications for existing cancer drugs.
Biovista Inc. - Specializing in AI-driven drug repositioning.
BenevolentAI - Leveraging deep learning for drug discovery.
Teva Pharmaceuticals - Leading in neurological drug repurposing.
The competitive landscape is expected to evolve as more companies adopt AI-based platforms and form strategic collaborations to expand their repurposing pipelines.
Future Outlook of the Drug Repurposing Market
The future of drug repurposing looks promising, with AI, big data, and personalized medicine driving innovation. Real-world data (RWD) and real-world evidence (RWE) are becoming integral to repurposing efforts, allowing companies to assess drug efficacy across different patient demographics.
Additionally, the rise of precision medicine is expected to further accelerate drug repurposing efforts, particularly in oncology and rare diseases. By identifying genetic markers and molecular targets, researchers can tailor treatments to specific patient populations, increasing therapeutic effectiveness.
With regulatory bodies streamlining approval pathways and AI optimizing drug discovery, drug repurposing will continue to play a crucial role in addressing global healthcare challenges.
Buy this Premium Research Report for exclusive, in-depth insights -
https://www.transparencymarketresearch.com/checkout.php?rep_id=59610<ype=S
Frequently Asked Questions (FAQs)
1. What is drug repurposing?
Drug repurposing, also known as drug repositioning, is the process of finding new therapeutic uses for existing drugs. This approach reduces development time and costs by leveraging previously approved medications for new disease indications.
2. How does drug repurposing benefit pharmaceutical companies?
Pharmaceutical companies benefit from drug repurposing as it lowers R&D costs, reduces failure rates, and accelerates market entry. Since repurposed drugs have established safety profiles, they can bypass early-stage clinical trials, increasing the likelihood of approval.
3. What role does AI play in drug repurposing?
Artificial intelligence (AI) enhances drug repurposing by analyzing big data, genetic information, and chemical interactions to identify potential drug-disease matches. AI-driven platforms like BenevolentAI and IBM Watson have been instrumental in accelerating discoveries.
4. What are some successful examples of drug repurposing?
Thalidomide - Originally a sedative, now used to treat multiple myeloma and leprosy.
Aspirin - Initially for pain relief, now widely used for cardiovascular disease prevention.
Sildenafil (Viagra) - Originally developed for hypertension, later repurposed for erectile dysfunction.
5. Which diseases are the primary focus for drug repurposing?
Key therapeutic areas include oncology, neurological disorders (Alzheimer's, Parkinson's), infectious diseases (HIV, COVID-19), and rare diseases, where treatment options are limited.
6. What are the challenges in the drug repurposing market?
While drug repurposing offers many advantages, challenges include intellectual property (IP) protection, patent expiration issues, and regulatory hurdles. Many repurposed drugs are off-patent, making it difficult for companies to secure market exclusivity.
7. How big is the drug repurposing market?
The market was valued at USD 28.9 billion in 2023 and is projected to reach USD 47.8 billion by 2034, growing at a CAGR of 4.7%.
8. What are the key trends shaping the future of drug repurposing?
The future of drug repurposing is driven by AI integration, big data analytics, personalized medicine, and regulatory fast-tracking. AI and machine learning will continue to play a crucial role in identifying new therapeutic applications, further expanding the market.
Explore Latest Research Reports by Transparency Market Research:
Blood Pressure Cuffs Market - https://www.transparencymarketresearch.com/blood-pressure-cuffs-market.html
Vaginal Slings Market - https://www.transparencymarketresearch.com/vaginal-slings-market.html
Stretcher Chairs Market - https://www.transparencymarketresearch.com/stretcher-chairs-market-2018-2026.html
Plasma Fractionation Market - https://www.transparencymarketresearch.com/plasma-fractionation-market.html
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
Email: sales@transparencymarketresearch.com
Follow Us: LinkedIn| Twitter| Blog | YouTube
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Drug Repurposing Market Set to Reach USD 47.8 Billion by 2034, Driven by Cost Efficiency and AI Integration |TMR here
News-ID: 3915626 • Views: …
More Releases from Transparency Market Research

Autoinjectors Market to be Worth USD 173.9 Bn by 2036 - By Usability / By Route …
The global autoinjectors market has emerged as a vital segment within the injectable drug delivery ecosystem, demonstrating robust expansion supported by technological innovation and the increasing prevalence of chronic diseases worldwide. The market was valued at US$ 89.9 billion in 2025 and is projected to reach US$ 173.9 billion by 2036, registering a compound annual growth rate (CAGR) of 6.2% from 2026 to 2036.
Preview crucial insights and findings from our…
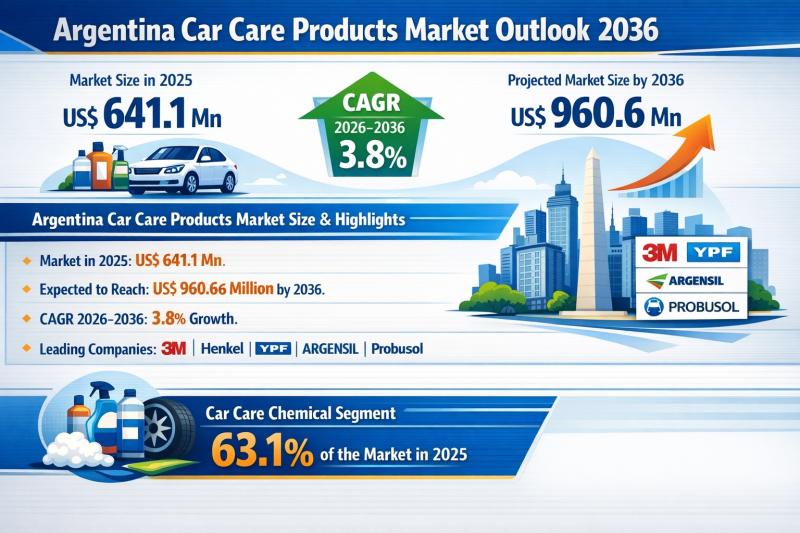
Argentina Car Care Products Market Outlook 2036: Market Size to Reach US$ 960.6 …
The Argentina car care products market continues to demonstrate steady, resilient growth, reflecting the country's large and aging vehicle fleet and rising consumer awareness around vehicle aesthetics and resale value. In 2025, the market was valued at US$ 641.1 million and is projected to expand to US$ 960.6 million by 2036, registering a compound annual growth rate (CAGR) of 3.8% from 2026 to 2036.
This growth trajectory underscores the essential nature…
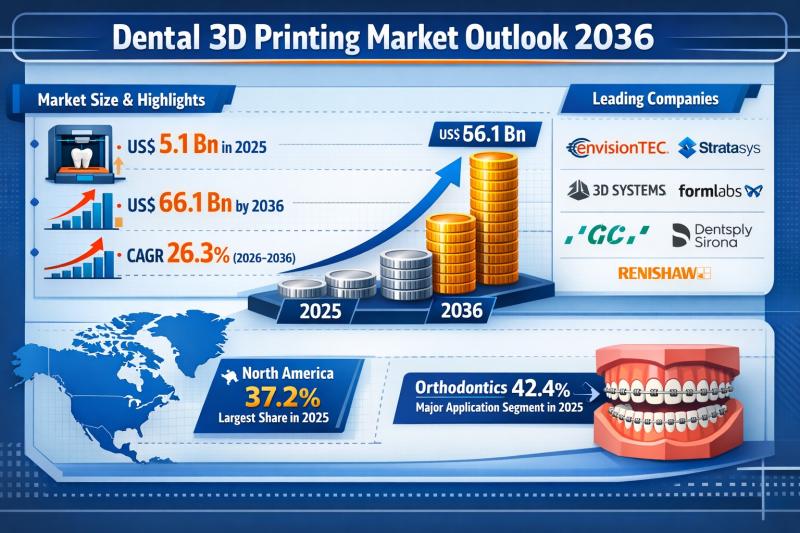
Dental 3D Printing Market Outlook 2036: Explosive 26.3% CAGR Growth Driven by Cu …
The global dental 3D printing market is entering a phase of extraordinary expansion. Valued at US$ 5.1 Bn in 2025, the market is projected to surge to US$ 66.1 Bn by 2036, registering a robust compound annual growth rate (CAGR) of 26.3% from 2026 to 2036. This rapid growth reflects the accelerating shift of dental practices and laboratories toward digital, patient-specific manufacturing solutions.
Such a steep growth curve positions dental 3D…
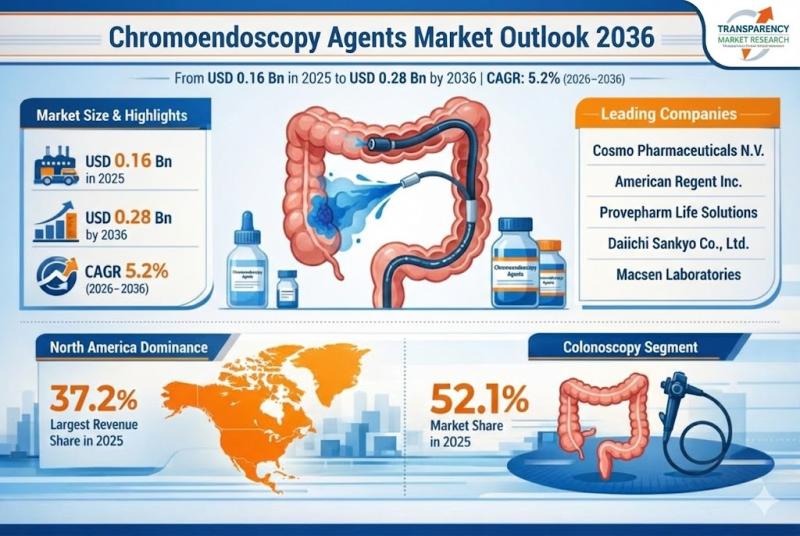
Chromoendoscopy Agents Market Outlook 2036: Rising GI Disorder Burden and Advanc …
The global chromoendoscopy agents market was valued at US$ 0.16 billion in 2025 and is projected to reach US$ 0.28 billion by 2036, expanding at a CAGR of 5.2% from 2026 to 2036. The market is witnessing consistent growth due to the rising prevalence of gastrointestinal (GI) disorders, increasing demand for early cancer detection, and growing adoption of advanced endoscopic diagnostic techniques in hospitals and specialty clinics worldwide.
Get Your Sample…
More Releases for Drug
Injectable Drug Delivery Market Injectable Drug Delivery Market
Leading market research firm SkyQuest Technology Group recently released a study titled ' Injectable Drug Delivery Market Global Size, Share, Growth, Industry Trends, Opportunity and Forecast 2024-2031,' This study Injectable Drug Delivery report offers a thorough analysis of the market, as well as competitor and geographical analysis and a focus on the most recent technological developments. The research study on the Injectable Drug Delivery Market extensively demonstrates existing and upcoming…
Global Advanced Drug Delivery Systems Market Size - By Product Type(Oral Drug De …
Market Overview and Report Coverage
Advanced Drug Delivery Systems (ADDS) refer to innovative technologies designed to improve the administration and efficacy of therapeutics, enhancing the way medications are delivered to targeted areas within the body. These systems aim to optimize treatment outcomes by increasing the bioavailability, reducing side effects, and facilitating controlled drug release. Employing methods such as nanoparticles, liposomes, and implantable pumps, ADDS are revolutionizing personalized medicine and expanding therapeutic…
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Pric …
Global Cancer Antibody Drug Conjugate Market Size, Drug Sales, Drug Dosage, Price, and Clinical Trials Outlook 2029 Report Highlights:
* Global Antibody Drug Conjugates Market Opportunity: > 40 Billion By 2029
* Global and Regional Antibody Drug Conjugate Market Insight
* Approved Drugs Sales Insight Global and Regional, Yearly and Quarterly, 2019 -2023
* Approved Antibody Drug Conjugates - Availability, Dosage and Price Insight
* Insight On Antibody Drug Conjugates In Clinical Trials: > 550…
Alcohol Testing And Drug Testing Equipment Market 2025 Segmentation, Application …
Market Study Report, LLC, has compiled an exhaustive research study of the ‘Alcohol Testing And Drug Testing Equipment market’, detailing every single market driver and intricately analyzing the business vertical. This ‘Alcohol Testing And Drug Testing Equipment market’ study will aid in seeking out new business opportunities and fine-tuning existing marketing strategies through insights regarding SWOT analysis, market valuation, competitive spectrum, regional share, and revenue predictions.
Alcohol abuse and drug…
How much Diabetes Drug Market Impact Worldwide Medical Drug Industry?
Diabetes Drug Market From an insight perspective, the market report focuses on various levels of analyses — industry analysis, market rank analysis, and company profiles, which together comprise and discuss basic views on the competitive landscape, high-growth regions, and countries as well as their respective regulatory policies, Types ,Applications and opportunities in the market.
Diabetes is a metabolic disorder in which the body glucose level is elevated. There are two types of diabetes…
Hepatitis Drug Market Hepatitis Drug Clinical Pipeline Report 2023
For Report Sample Contact: neeraj@kuickresearch.com or +91-11-47067990
Report Table of Contents
1. Introduction to Hepatitis Disease
1.1 Prologue
1.1.1 History of Hepatitis
1.1.2 Causes of Hepatitis Disease
1.2 Types of Viruses which are Responsible for Hepatitis Disease
2. Global Prevalence of Hepatitis Infection
3. Available Drug Classes for Hepatitis Disease Treatment
3.1 Interferon Alfa Therapy
3.2 Protease Inhibitors Therapy
3.3 Polymerase…
