Press release
Biosimilar Market Analysis, Booming at 17.02% CAGR and $74.7B by 2030
Biosimilar Market is anticipated to reach around USD 74.70 billion by 2030, with a compound annual growth rate CAGR of 17.02 percent from 2024 to 2030. Biosimilar & Follow-on Biologics Market expected to witness a modest growth with estimated compound annual growth rate over the forecast period. The report notes that the most significant drivers behind this growth include demand for low-cost biologic alternatives, the patent expiration of prominent biologic medications, and the rising incidence of chronic disease. Key market trends --The increasing use of biosimilars, as cost-effective alternatives to branded biologics, is also driving the growth of the market.Request a sample link for more details : https://www.maximizemarketresearch.com/request-sample/83592/
Reasons for Market Growth & Opportunities
The withering of biosimilar and follow-on biologics market is cleansing towards a progression stage, addressing the demand for effective and affordable health and nutrition solution, on the influence of progressively benefiting healthy diet and the growing prevalence of chronic diseases including cancer, diabetes, along with autoimmune diseases among others. These products are highly similar to approved biologics and they have cost-effectiveness, making them an appealing option for healthcare providers and patients. The patents on many blockbuster biologic drugs are due to expire, providing significant opportunities for biosimilar manufacturers to enter the market.
Market growth is also the result of government initiatives and favorable regulatory policies. This is due to the fact that there are regulatory agencies like the U.S. Food and Drug Administration and the European Medicines Agency with well-defined streamlined approval pathways to facilitate the development and commercialization of biosimilars. Moreover, the growing emphasis on cost containment in healthcare and the need for patient access to biologic therapy is fueling the global uptake of biosimilars.
Segmentation Analysis
The market for biosimilars and follow-on biologics is segmented on the basis of product type, application, and region. Based on product type the market is segmented into recombinant non-glycosylated proteins, recombinant glycosylated proteins, and recombinant peptides. Recombinant glycosylated proteins lead the market, due to their high application in the treatment of chronic diseases including cancer and autoimmune diseases.
Would you like to access more information ? The process begins with the sample request : https://www.maximizemarketresearch.com/request-sample/83592/
On the basis of application, the market is segmented into oncology, chronic and autoimmune diseases, growth hormone deficiency, infectious diseases, and others. By indication, the oncology segment accounted for the largest market share, primarily due to the high prevalence of cancer and the increased adoption and availability of biosimilars related to the treatment of cancer. By application, the chronic and autoimmune diseases segment is estimated to experience the highest CAGR, driven by the increasing prevalence of rheumatoid arthritis, diabetes, and other diseases.
Geographical Segmentation North America, Europe, Asia-Pacific, Latin America, Middle East & Africa Europe revels in the market, owing to the availability of a robust regulatory framework coupled with the increased adoption of biosimilars. The Asia-Pacific region is expected to grow at the fastest rate, propelled by rising healthcare spending and the growing need for BIOSIMILARS in emerging economies such as China and India.
Country-Level Analysis
United States: The U.S. is an important player in the biosimilar and follow-on biologics market, owing to the high prevalence of chronic diseases and the presence of major pharmaceutical companies. The country is also seeing major investments in biosimilar development, combined with government policies that are conducive to this industry.
Germany led the way among European countries in fostering the biosimilar market, focusing on both cost savings in healthcare and the affordability of billions of patients needing biologic therapies. A well-established healthcare infrastructure and a favorable regulatory environment are also the key growth drivers in the country.
China: China is one of the largest pharmaceutical market and a key part of the biosimilar industry. Rapid urbanization, combined with an increasing demand for cost-effective healthcare solutions, is contributing to the growth of biosimilars in the country.
India: India is rising as a noteworthy and lucrative market for biosimilars with the growing burden of chronic ailments and the government's emphasis on lowering healthcare expenditures. It also further reflecting growth in the market is the country's growing pharmaceutical industry and favoring regulatory policies.
Japan: Japan is one of the key markets for biosimilars owing to high pervasiveness of chronic diseases & increasing acceptance of biosimilars for cancer treatment. Key growth drivers include the country's well-established healthcare system and favorable regulatory environment.
To get more information about the market analysis , check out the research report summary : https://www.maximizemarketresearch.com/market-report/global-biosimilar-and-follow-on-biologics-market/83592/
Competitor Analysis
The competition is high in the biosimilar and follow-on biologics market due to presence of couple of key players fighting for market share. Top companies: Novartis AG, Pfizer Inc., Amgen Inc., Biocon Limited, Celltrion Inc These market players are engaged in strategic partnerships, product innovations, and merger and acquisitions to enhance their market position.
Biossoftware products by Novartshire AG are available only through Authorized Novartis vehicles/partners. Recent advances in biosimilar development have cemented the company's leadership.
Pfizer Inc is a leader in biosimilars, with a particular market focus on oncology and autoimmune diseases. By entering into strategic partnerships and investing in R&D, the company has continued to stay strong in the market.
Biopharmaceutical company Amgen Inc. is expanding its biosimilar pipeline by utilizing its biologic drug development know-how. Its innovation and strategic partnerships have also expanded market reach.
Celltrion Inc. is concentrating on expanding its biosimilar line-up, notably for oncology and immune disorders. Strategic acquisitions and R&D investments by the company have improved its technical capabilities and market footprint.
Press Release Conclusion
Increased chronic diseases and the demand for effective biologic treatments will keep growing the global biosimilar and follow-on biologics market. The report provides a detailed overview of the market with respect to the competition landscape, key players, and regional developments. The future appears bright for biosimilars, an active and ever-changing market, as healthcare systems worldwide keep supporting their use as cost-effective alternatives to proprietary biologics.
For further information, please visit :
Global Wound Dressings Market https://www.maximizemarketresearch.com/market-report/global-wound-dressings-market/21036/
Pre Clinical Imaging Market https://www.maximizemarketresearch.com/market-report/global-pre-cinical-imaging-market/8476/
Contact Maximize Market Research:
MAXIMIZE MARKET RESEARCH PVT. LTD.
⮝ 3rd Floor, Navale IT park Phase 2,
Pune Banglore Highway, Narhe
Pune, Maharashtra 411041, India.
✆ +91 9607365656
sales@maximizemarketresearch.com
www.maximizemarketresearch.com
About Maximize Market Research:
Maximize Market Research is one of the fastest-growing market research and business consulting companies. Most Fortune 500 organizations are pleased to join with us because of our revenue impact and targeted growth-driven research projects. Our portfolio is diverse and we work with a range of industries, including healthcare, chemical, food and beverage, IT and telecom, and aerospace and defense.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Biosimilar Market Analysis, Booming at 17.02% CAGR and $74.7B by 2030 here
News-ID: 3915287 • Views: …
More Releases from MAXIMIZE MARKET RESEARCH PVT. LTD
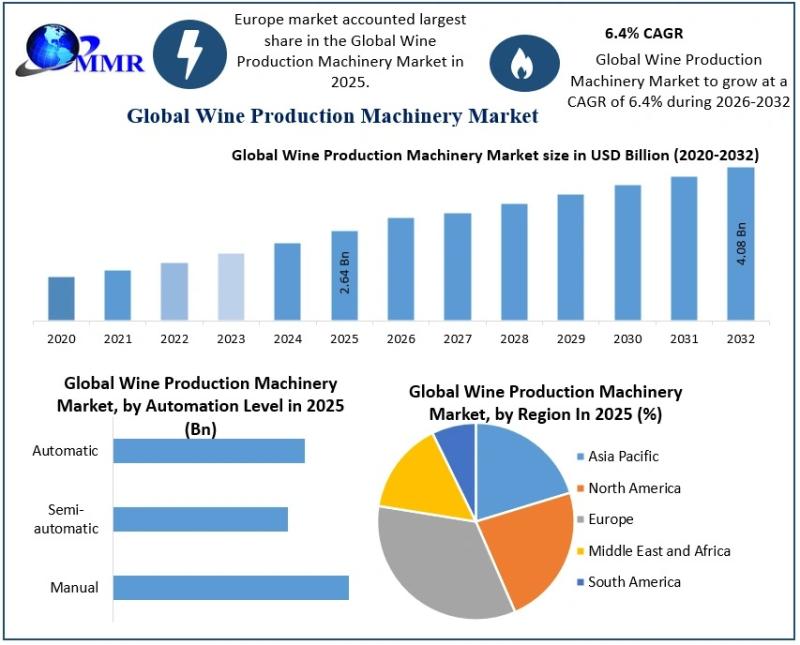
Wine Production Machinery Market Growing at a Robust CAGR of 6.4% Driven by Auto …
The Wine Production Machinery Market size was valued at USD 2.64 Billion in 2025 and the total Wine Production Machinery revenue is expected to grow at a CAGR of 6.4% from 2026 to 2032, reaching nearly USD 4.08 Billion by 2032.
Wine Production Machinery Market Overview:
The Wine Production Machinery Market is witnessing steady transformation as wineries across the globe increasingly adopt modern equipment to enhance efficiency, consistency, and product quality. From…
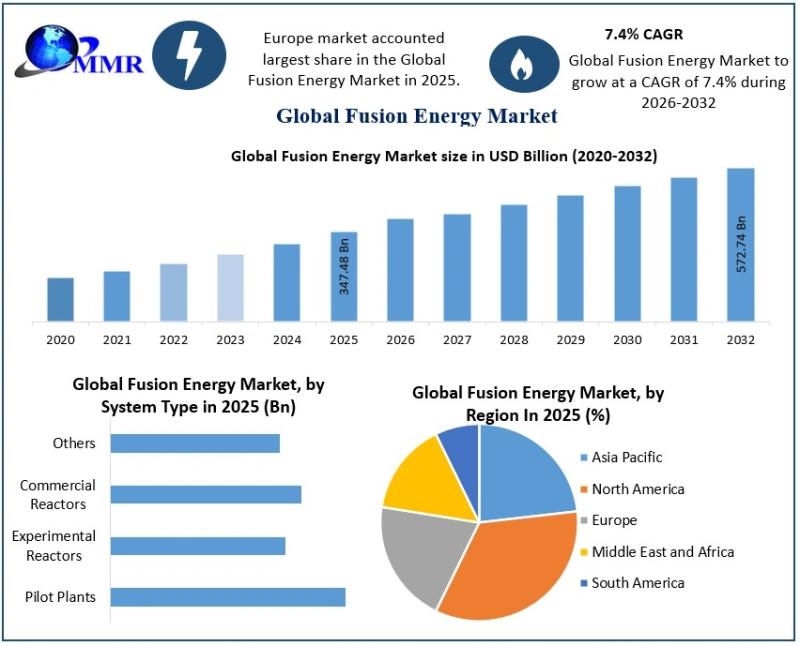
Fusion Energy Market Outlook Highlights Strong Growth at 7.4% CAGR
The Fusion Energy Market size was valued at USD 347.48 Billion in 2025 and the total Fusion Energy revenue is expected to grow at a CAGR of 7.4% from 2026 to 2032, reaching nearly USD 572.74 Billion by 2032.
Fusion Energy Market Overview:
The Fusion Energy Market is gaining global attention as nations, industries, and researchers intensify efforts to develop cleaner and more sustainable power alternatives for the future. Fusion energy is…
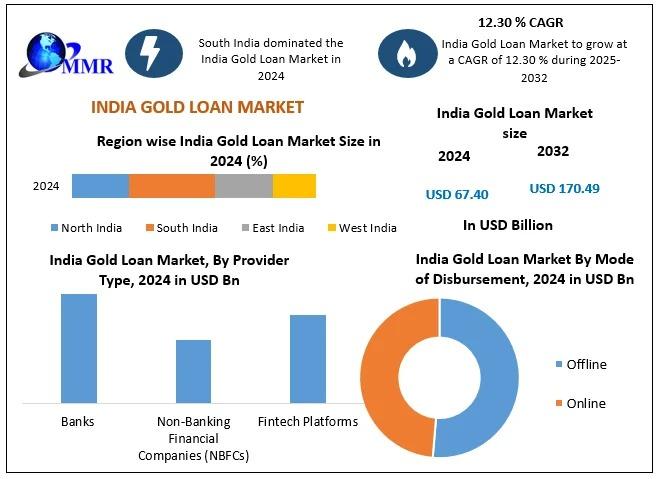
India Gold Loan Market Shows Strong Momentum Driven by Trust, Technology, and Fi …
The India Gold Loan Market size was valued at USD 67.40 Billion in 2024 and the total India Gold Loan Market is expected to grow at a CAGR of 12.30 % from 2025 to 2032, reaching nearly USD 170.49 Billion.
India Gold Loan Market Overview:
The India Gold Loan Market has steadily evolved into a trusted financial solution for individuals and small businesses seeking quick access to funds without liquidating their long-term…
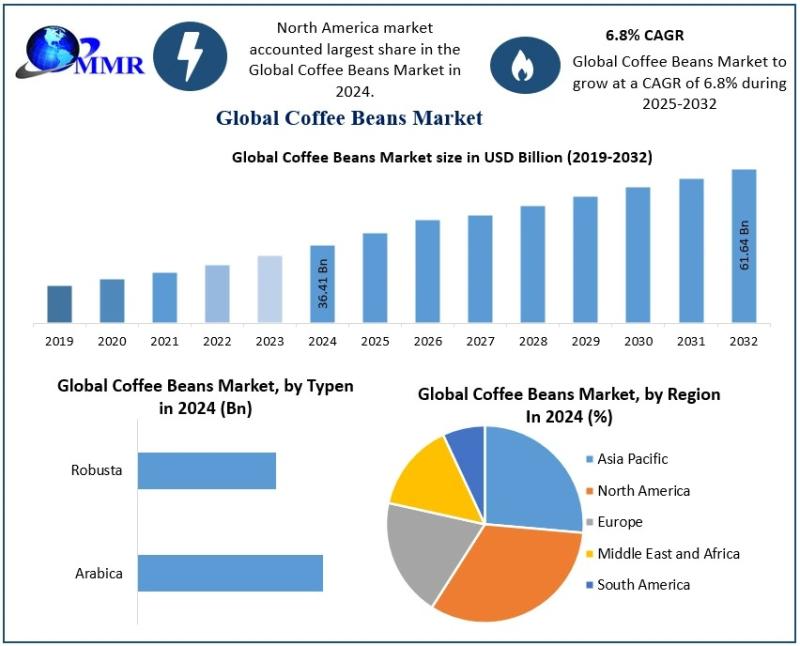
Coffee Beans Market Trends Highlight Rising Demand for Specialty, Sustainable, a …
The Coffee Beans Market size was valued at USD 36.41 Billion in 2024 and the total Coffee Beans revenue is expected to grow at a CAGR of 6.8% from 2025 to 2032, reaching nearly USD 61.64 Billion.
Coffee Beans Market Overview:
The Coffee Beans Market reflects a complex ecosystem that begins at farms and extends to global distribution networks. Coffee beans are cultivated across diverse climatic regions, each contributing unique taste profiles…
More Releases for Biosimilar
Interchangeable Biosimilar Humira Market Share Driven by Biologic Therapy Adopti …
Interchangeable Biosimilar Humira Market
The global market for Interchangeable Biosimilar Humira was valued at US$ million in the year 2024 and is projected to reach a revised size of US$ million by 2031, growing at a CAGR of %during the forecast period
View sample report
https://reports.valuates.com/request/sample/QYRE-Auto-33I15005/Global_Interchangeable_Biosimilar_Humira_Market_Research_Report_2023
The Interchangeable Biosimilar Humira Market is experiencing significant market growth as healthcare providers and patients increasingly adopt biosimilar therapies for autoimmune and inflammatory conditions. Market trends indicate rising…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Key Trend Reshaping the Biosimilar Monoclonal Antibodies Market in 2025: Advance …
What Are the Projections for the Size and Growth Rate of the Biosimilar Monoclonal Antibodies Market?
In recent times, the biosimilar monoclonal antibodies sector has experienced a swift expansion. The market size, which stands at $8.04 billion in 2024, is projected to climb to $9.25 billion in 2025, marking a compound annual growth rate (CAGR) of 15.1%. Factors such as expired patents, an increased understanding of biosimilars, governmental strategies, heightened financial…
Biosimilar Market Treating More for Less: The Booming Infliximab Biosimilar Mark …
Infliximab Biosimilar Market worth $ XX Million by 2030 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Infliximab Biosimilar Market- by Application (Crohn's Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis and Others), End User (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Other Direct Distribution Channels), Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get…
Biosimilar Monoclonal Antibodies Market
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the " "Global Biosimilar Monoclonal Antibodies Market by Product (infliximab, trastuzumab, rituximab, adalimumab, bevacizumab, cetuximab, ranibizumab, denosumab, eculizumab, and other pipeline products), Indication (oncology, inflammatory & autoimmune disorders, chronic diseases, blood disorders, and other indications), Clinical Trial/Pipeline Analysis, Future Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
The Biosimilar Monoclonal Antibodies Market Size is valued at 5.02…
Infliximab Biosimilar Insight, 2022 | DelveInsight
DelveInsight's, "Infliximab Biosimilar Insight, 2022" report provides comprehensive insights about 35+ companies and 45+ marketed and pipeline drugs in Infliximab Biosimilars landscape. It covers the marketed and pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Interested to know more about the functioning of…
