Press release
Rubber Oligomer Impurity Standards: Ensuring Medical-Grade Purity by Overcoming Impurity Challenges in Healthcare Applications to Reduce Contamination Risks, and Meet Stringent Regulatory Requirements
Rubber Oligomer Impurity Standards play a pivotal role in ensuring the highest level of purity in medical and pharmaceutical applications. As rubber-based materials are widely used in medical devices, drug packaging, and laboratory equipment, the presence of impurities can pose serious health risks. To maintain the integrity and biocompatibility of these materials, it is essential to monitor and control Rubber Oligomers that may lead to contamination or adverse biological reactions.Rubber-based products in the healthcare industry are subjected to various environmental factors such as heat, moisture, and chemical exposure. These conditions can accelerate the degradation of rubber, leading to the release of oligomeric impurities. If not identified and controlled, these impurities can compromise the effectiveness of medical devices and pharmaceuticals, leading to potential health hazards. Therefore, stringent impurity testing and adherence to regulatory guidelines are critical for manufacturers.
Rubber Oligomer Impurities and Their Impact
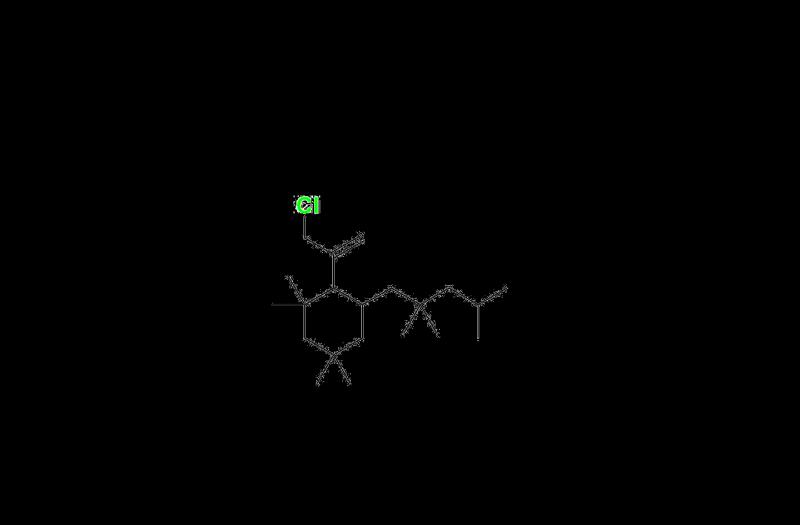
Rubber Oligomers are low-molecular-weight compounds formed during the polymerization and degradation of rubber materials. While they contribute to the flexibility and durability of rubber-based products, uncontrolled levels of these oligomers can introduce impurities that compromise product safety and regulatory compliance. Impurities such as 1-(3,5-di-tert-butyl-4-hydroxyphenyl)ethan-1-one and 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoic acid have been identified as critical markers in impurity analysis, necessitating stringent testing and standardization.
Rubber oligomer impurities can lead to significant challenges in healthcare applications. For example, in pharmaceutical packaging, impurities can interact with drugs, altering their stability and efficacy. In medical devices, these impurities can cause material degradation, reducing the lifespan of products such as catheters, syringes, and gloves. Therefore, maintaining high purity levels in rubber-based products is essential for ensuring patient safety and product reliability.
To know more about Rubber Oligomers : - https://aquigenbio.com/products/impurity-standards/rubber-oligomers/
The Importance of Rubber Oligomer Impurity Standards in Regulatory Compliance
Regulatory bodies such as the FDA and EMA impose strict guidelines on the purity of materials used in healthcare applications. Rubber Oligomer Impurity Standards are essential for ensuring compliance with these stringent regulations. By accurately identifying, quantifying, and controlling Rubber Oligomers, manufacturers can enhance product safety, prevent contamination, and meet industry standards for biocompatibility.
Failure to comply with regulatory requirements can result in product recalls, legal actions, and reputational damage for manufacturers. To mitigate these risks, companies must implement rigorous testing protocols and adopt industry-approved Rubber Oligomer Impurity Standards. This ensures that medical and pharmaceutical products remain free from harmful contaminants, safeguarding patient health and regulatory compliance.
Enhancing Biocompatibility and Reducing Contamination Risks
Biocompatibility is a key concern in medical applications where rubber components come in direct contact with patients, such as in intravenous tubing, surgical gloves, and pharmaceutical packaging. Uncontrolled Rubber Oligomer impurities can lead to cytotoxicity, allergic reactions, or chemical leaching, ultimately affecting patient safety. Implementing Rubber Oligomer Impurity Standards ensures that only medical-grade materials with minimal contamination risks are used in healthcare products.
By adhering to strict impurity standards, manufacturers can ensure that rubber-based products meet the highest biocompatibility requirements. This is particularly important for medical implants and devices that remain inside the human body for extended periods. Any impurity-related complications could lead to severe health issues, emphasizing the necessity of rigorous quality control measures.
Advanced Analytical Techniques for Rubber Oligomer Detection
Modern analytical techniques, such as High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS), enable precise detection and quantification of Rubber Oligomers. These methods provide reliable data for impurity profiling, allowing manufacturers to refine their materials and maintain consistency in product quality. The identification of specific impurities like 1-(3,5-di-tert-butyl-4-hydroxyphenyl)ethan-1-one and 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoic acid and Rubber Oligomer- 4 further strengthens quality control measures.
Additional advanced techniques such as Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR) spectroscopy provide highly sensitive methods for detecting trace impurities in rubber materials. By utilizing these cutting-edge analytical tools, manufacturers can develop more refined and effective quality control processes, ensuring compliance with international safety standards.
Explore about Rubber Oligomer-4: https://aquigenbio.com/product/rubber-oligomer-4/
Conclusion
Ensuring the highest level of purity in healthcare applications is critical, and maintaining compliance with Rubber Oligomer Impurity Standards is essential. With advanced analytical solutions and regulatory support, manufacturers can achieve superior biocompatibility and uphold the highest quality standards. As regulations evolve, staying ahead in impurity detection and control ensures that healthcare products meet stringent safety and performance benchmarks. In future, the demand for robust Rubber Oligomer Impurity Standards will increase. Researchers and industry experts are continuously developing more refined analytical methods to detect even trace amounts of Rubber Oligomers, ensuring unparalleled purity and safety. The advancement in material science and regulatory frameworks will further drive the standardization of rubber-based medical components.
To know more about 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoic acid : -https://aquigenbio.com/product/3-35-di-tert-butyl-4-hydroxyphenylpropanoic-acid-impurity/
Contact:
Aquigen Bio Sciences
281/1, Plot No 41,
Hinjawadi - Pirangut Rd,
Kasar Amboli, Pirangut,
Pune, Maharashtra 412108
Phone: +91 7030123794
Email: bd@aquigenbio.com
Visit: www.aquigenbio.com
About Aquigen Bio Sciences
Aquigen Bio Sciences is a leading provider of Rubber Oligomer Impurity Standards in India, offering cutting-edge analytical solutions to ensure the highest levels of purity and compliance in healthcare applications. With a strong foundation in scientific research and regulatory expertise, the company helps manufacturers detect, analyze, and control impurities in rubber-based medical products. Committed to excellence, Aquigen Bio Sciences supports the healthcare and pharmaceutical industries by providing precise impurity standards that enhance product safety and biocompatibility. Their innovative approach and stringent quality control measures make them a trusted partner for manufacturers striving to meet global regulatory requirements and uphold the integrity of medical-grade materials.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Rubber Oligomer Impurity Standards: Ensuring Medical-Grade Purity by Overcoming Impurity Challenges in Healthcare Applications to Reduce Contamination Risks, and Meet Stringent Regulatory Requirements here
News-ID: 3908295 • Views: …
More Releases from Aquigen Bio Sciences

Elevate Pharmaceutical R&D with Aquigen BioSciences' Precision‐Grade Flibanser …
Flibanserin Impurity B is a reference standard used in pharmaceutical research and development. It is primarily applied during the analysis and validation of drug substances to identify, quantify, and control impurities that may be present in the final product. This impurity is associated with the parent compound, Flibanserin, a medication approved for the treatment of hypoactive sexual desire disorder (HSDD) in premenopausal women.
Aquigen BioSciences offers Flibanserin Impurity B as a…

Estradiol Valerate EP Impurity A - Premium Reference Standard for Analytical Dev …
Estradiol Valerate EP Impurity A is a high-quality reference standard designed to meet the stringent requirements of pharmaceutical research, method validation, and quality control processes.
Explore Estradiol Valerate EP Impurity A :
https://aquigenbio.com/product/estradiol-valerate-ep-impurity-a/
Manufactured and characterized with precision, this impurity standard supports laboratories and manufacturers in achieving consistent, reliable, and reproducible results in critical analytical workflows.
With its exceptional purity and accurate characterization, Estradiol Valerate EP Impurity A plays a vital role…

High-Purity N-Nitroso Betahistine D3 for Precise Pharmaceutical Analysis | Deute …
Product Overview
N-Nitroso Betahistine D3 is a premium deuterated nitrosamine impurity standard, specifically developed for precise analytical testing in pharmaceutical laboratories. This reference standard is widely used for analytical method development, validation, and quality control processes to meet stringent regulatory guidelines. With exceptional purity, complete documentation, and reliable traceability, it is ideal for research, development, and compliance applications.
https://aquigenbio.com/product/n-nitroso-betahistine-d3/
Key Features and Benefits
Deuterated Design for Precision: The incorporation of deuterium improves mass spectrometric…

Aquigen Bio Strengthens Pharmaceutical Research with High-Purity Icatibant Impur …
Aquigen Bio, a trusted supplier of pharmaceutical reference standards, today announced the expansion of its Icatibant Impurity Standards portfolio, designed to support drug developers, analytical laboratories, and research organizations with reliable materials for impurity profiling and quality control.
Icatibant, a selective bradykinin B2 receptor antagonist, is widely used in the treatment of hereditary angioedema (HAE). Given its peptide-based structure, Icatibant is prone to the formation of impurities during synthesis and storage.…
More Releases for Rubber
Stationery Rubber Bands Market Size Set for Rapid Growth and Trend by 2030 | Dyk …
Global "Stationery Rubber Bands Market" Research report is an in-depth study of the market Analysis. Along with the most recent patterns and figures that uncovers a wide examination of the market offer. This report provides exhaustive coverage on geographical segmentation, latest demand scope, growth rate analysis with industry revenue and CAGR status. While emphasizing the key driving and restraining forces for this market, the report also offers a complete study…
Rubber Mulch Market Promising Regions for Companies in 2023 | Rubber Mulch Produ …
The global Rubber Mulch market is thoroughly researched in this report, noting important aspects like market competition, global and regional growth, market segmentation, and market structure. Our team of analysts has employed the latest research tools and techniques to estimate the size of the Rubber Mulch market in terms of both value and volume. Furthermore, this report includes detailed estimates for market share, revenue, production, consumption, gross profit margin, CAGR…
Reclaimed Rubber and Rubber Powder Market to Witness Huge Growth by Key Players: …
The Reclaimed Rubber and Rubber Powder report compiles the market information depending upon market development and growth factors, optimizing the growth path. In addition, it highlights the strategies and market share of the leading vendors in the particular market. The report follows a robust research methodology model that helps to make informed decisions. It obtains both qualitative and quantitative market information supported by primary research.
The Reclaimed Rubber and Rubber Powder…
Pakistan Rubber Tyre Market : Pneumatic Rubber Tyre, Retreaded Rubber Tyre, Cush …
According to a recent report published by Allied Market Research, titled, "Pakistan rubber tyre Market by Tyre, Component, Design, and Vehicle Type: Opportunity Analysis and Industry Forecast, 2018 - 2025," Pakistan rubber tyre market size was valued at $272.10 million in 2017, and is projected to reach $1,592.90 million by 2025, registering a CAGR of 24.8% from 2018 to 2025. The radial type design segment was the highest contributor to…
Pakistan Rubber Tyre Market : Pneumatic Rubber Tyre, Retreaded Rubber Tyre, Cush …
The global Pakistan rubber tyre market size was valued at $272.10 million in 2017, and is projected to reach $1,592.90 million by 2025, registering a CAGR of 24.8% from 2018 to 2025. The radial type by design segment was the highest revenue contributor in 2017, accounting for $207.7 million, and is estimated to reach $1,196.4 million by 2025, registering a CAGR of 24.6% during the forecast period.
Download Sample Report at…
Pakistan Rubber Tyre Market : Pneumatic Rubber Tyre, Retreaded Rubber Tyre, Cush …
According to a recent report published by Allied Market Research, titled, "Pakistan rubber tyre Market by Tyre, Component, Design, and Vehicle Type: Opportunity Analysis and Industry Forecast, 2018 - 2025," Pakistan rubber tyre market size was valued at $272.10 million in 2017, and is projected to reach $1,592.90 million by 2025, registering a CAGR of 24.8% from 2018 to 2025. The radial type design segment was the highest contributor to…