Press release
Osteosarcoma Clinical Trials and Studies: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies
DelveInsight's, "Osteosarcoma Pipeline Insight 2025" report provides comprehensive insights about 25+ companies and 30+ pipeline drugs in Osteosarcoma pipeline landscape. It covers the Osteosarcoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Osteosarcoma pipeline therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Stay ahead in the competitive pharmaceutical landscape with in-depth Pipeline Insights! Discover the latest advancements, key Osteosarcoma Companies driving innovation, and the most promising Emerging Drugs shaping the future of treatment. Download our comprehensive report now to explore clinical-stage developments and strategic collaborations transforming the industry! @ Osteosarcoma Pipeline Outlook Report [https://www.delveinsight.com/sample-request/osteosarcoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Osteosarcoma Pipeline Report
* In February 2025:- Ipsen :- The participants of this study will be children, adolescents, and young adults with residual osteosarcoma, which cannot be removed completely through surgery. Participants will have achieved a partial response or stable disease at the end of conventional chemotherapy. Osteosarcoma is cancer of the bone. The cancer cells make immature bone cells, known as osteoid.
* In February 2025:- Adaptimmune :- This is a pediatric basket study to investigate the safety and efficacy of afamitresgene autoleucel in HLA-A*02 eligible and MAGE-A4 positive subjects aged 2-21 years of age with advanced cancers.
* In February 2025:- Sarcoma Oncology Research Center LLC - This study investigates the safety/toxicity and potential anti-tumor activity of sequential administration of nivolumab and escalating doses of the mammalian target of rapamycin (mTOR) inhibitor nab-rapamycin (ABI-009) in advanced Ewing's sarcoma, perivascular epithelioid cell tumor (PEComa), epithelioid sarcoma, desmoid tumor, chordoma, non-small cell lung cancer, small cell lung cancer, urothelial carcinoma, melanoma, renal cell carcinoma, squamous cell carcinoma of head and neck, hepatocellular carcinoma, classical Hodgkin's lymphoma, high microsatellite instability (MSI-H)/ mismatch repair deficient (dMMR) metastatic colorectal cancer, and tumors with genetic mutations sensitive to mTOR inhibitors.
* In February 2025:- Eli Lilly and Company :- The purpose of this study is to measure the benefit of adding abemaciclib to chemotherapy (irinotecan and temozolamide) for Ewing's sarcoma that has come back or did not respond to treatment. This trial is part of the CAMPFIRE master protocol, which is a platform to speed development of new treatments for children and young adults with cancer. Your participation in this trial could last 11 months or longer, depending on how you and your tumor respond.
* In February 2025: Merck Sharp & Dohme LLC :- Substudy 01A is part of a platform study. The purpose of this study is to assess the efficacy and safety of zilovertamab vedotin in pediatric participants with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL), diffuse large B-cell lymphoma (DLBCL)/Burkitt lymphoma, or neuroblastoma and in pediatric and young adult participants with Ewing sarcoma.
* DelveInsight's Osteosarcoma pipeline report depicts a robust space with 25+ active players working to develop 30+ pipeline therapies for Osteosarcoma treatment.
* The leading Osteosarcoma Companies such as Zentalis Pharmaceuticals, MedPacto, Salarius Pharmaceuticals, NextPoint Therapeutics, Base Therapeutics, EMD Serono, Hansoh BioMedical R&D, Cellectar Biosciences, OS Therapies, AlaMab Therapeutics , and others.
* Promising Osteosarcoma Therapies such as Olaparib, Ceralasertib, Vactosertib, Avelumab, ZN-c3, Gemcitabine and others.
Uncover Osteosarcoma groundbreaking developments in the drug pipeline with our latest report! Gain exclusive insights into Osteosarcoma Clinical Trials, regulatory updates, and Emerging Drugs across multiple therapeutic areas. Learn how leading Osteosarcoma Companies are positioning themselves for success in the evolving pharmaceutical market-access the full report today!" @ Osteosarcoma Clinical Trials Assessment [https://www.delveinsight.com/sample-request/osteosarcoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Osteosarcoma Emerging Drugs Profile
* HS-20093: Hansoh BioMedical R&D Company
HS-20093 is a novel B7-H3-targeted antibody-drug conjugate that consists of a fully humanized anti-B7-H3 monoclonal antibody covalently linked to a topoisomerase inhibitor (TOPOi) payload. This innovative drug is being developed for the treatment of lung cancer, sarcoma, head and neck cancers, and other solid tumors through multiple phase I and II clinical trials in China. The mechanism of action (MOA) of HS-20093 involves the specific targeting of B7-H3, a transmembrane receptor highly prevalent on malignant cells, with the monoclonal antibody component. This targeting allows for the delivery of the topoisomerase inhibitor payload directly to the cancer cells, where it can exert its cytotoxic effects, potentially leading to tumor cell death and inhibition of tumor growth. Currently, the drug is in Phase II stage of its clinical trial for the treatment of Osteosarcoma.
* ZN c3: Zentalis Pharmaceuticals
ZN-c3 is a combination with gemcitabine used in adult and pediatric subjects with relapsed or refractory osteosarcoma. ZN-c3 is a phase I/II drug, developed by Zentalis Pharmaceuticals. ZN-c3 drug is an investigational drug and gemcitabine is an approved drug. ZN-c3 have received a fast track designation for uterine cancer but is under phase I/II for osteosarcoma.
* NPX267: Nextpoint Therapeutics
NPX267 is a first-in-class monoclonal antibody targeting KIR3DL3, the inhibitory receptor for the HHLA2 pathway (B7-H7), and is designed to prevent immune escape in solid tumors. By blocking the binding of KIR3DL3 on exhausted T and NK cells to HHLA2 expressed on tumor cells, NPX267 may be able to reactivate tumor antigen-primed immune cells in HHLA2-expressing solid tumors. Preventing KIR3DL3-mediated immune suppression may enhance anti-tumor immune responses for patients with HHLA2-positive cancers - a tumor antigen expressed independently of PD-L1. Currently, the drug is in Phase I stage of its clinical trial for the treatment of Osteosarcoma.
The Osteosarcoma pipeline report provides insights into
* The report provides detailed insights about companies that are developing therapies for the treatment of Osteosarcoma with aggregate therapies developed by each company for the same.
* It accesses the Different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of development for Osteosarcoma Treatment.
* Osteosarcoma Companies are involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
* Osteosarcoma Drugs under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
* Detailed analysis of collaborations (company-company collaborations and company-academia collaborations), licensing agreement and financing details for future advancement of the Osteosarcoma market.
Explore the dynamic world of drug development with our latest Osteosarcoma Pipeline Insights report! From early-stage research to late-phase Osteosarcoma Clinical Trials, our analysis covers key Osteosarcoma Companies, innovative treatment approaches, and the next wave of Emerging Drugs. Don't miss this opportunity to stay informed-download now! @ Osteosarcoma Treatment Drugs [https://www.delveinsight.com/sample-request/osteosarcoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Osteosarcoma Companies
Zentalis Pharmaceuticals, MedPacto, Salarius Pharmaceuticals, NextPoint Therapeutics, Base Therapeutics, EMD Serono, Hansoh BioMedical R&D, Cellectar Biosciences, OS Therapies, AlaMab Therapeutics, and others.
Osteosarcoma pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as,
* Oral
* Intravenous
* Subcutaneous
* Parenteral
* Topical
Osteosarcoma Products have been categorized under various Molecule types such as
* Recombinant fusion proteins
* Small molecule
* Monoclonal antibody
* Peptide
* Polymer
* Gene therapy
The future of medicine is evolving rapidly! Get detailed insights into ongoing Osteosarcoma Clinical Trials, major Companies driving innovation, and the most anticipated Emerging Drugs in the pipeline. Stay updated with the latest Osteosarcoma Pipeline Insights-download our report for a deep dive into the next generation of therapeutics! @ Osteosarcoma Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/osteosarcoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Osteosarcoma Pipeline Report
* Coverage- Global
* Osteosarcoma Companies- Zentalis Pharmaceuticals, MedPacto, Salarius Pharmaceuticals, NextPoint Therapeutics, Base Therapeutics, EMD Serono, Hansoh BioMedical R&D, Cellectar Biosciences, OS Therapies, AlaMab Therapeutics, and others.
* Osteosarcoma Therapies- Olaparib, Ceralasertib, Vactosertib, Avelumab, ZN-c3, Gemcitabine and others.
* Osteosarcoma Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Osteosarcoma Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Which Osteosarcoma Emerging Drugs are set to revolutionize treatment landscapes? Which Osteosarcoma Companies are leading the way in drug discovery? Find answers in our latest Osteosarcoma Pipeline Insights report, featuring in-depth coverage of Clinical Trials, regulatory trends, and upcoming breakthroughs. Download now to stay at the forefront of pharmaceutical innovation!" @ Osteosarcoma Emerging Drugs and Companies [https://www.delveinsight.com/sample-request/osteosarcoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Osteosarcoma: Overview
* Osteosarcoma Pipeline Therapeutics
* Osteosarcoma Therapeutic Assessment
* Osteosarcoma - DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* Drug name: Company name
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* HS-20093: Hansoh BioMedical R&D Company
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I/II)
* NPX267: Nextpoint Therapeutics
* Drug profiles in the detailed report.....
* Inactive Products
* Osteosarcoma Key Companies
* Osteosarcoma Key Products
* Osteosarcoma- Unmet Needs
* Osteosarcoma- Market Drivers and Barriers
* Osteosarcoma- Future Perspectives and Conclusion
* Osteosarcoma Analyst Views
* Osteosarcoma Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=osteosarcoma-clinical-trials-and-studies-ema-pdma-fda-approvals-mechanism-of-action-roa-nda-ind-and-companies]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/report-store/medical-marijuana-market-insight
Legal Disclaimer: Information contained on this page is provided by an independent third-party content provider. ABNewswire makes no warranties or responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you are affiliated with this article or have any complaints or copyright issues related to this article and would like it to be removed, please contact retract@swscontact.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Osteosarcoma Clinical Trials and Studies: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies here
News-ID: 3897256 • Views: …
More Releases from ABNewswire
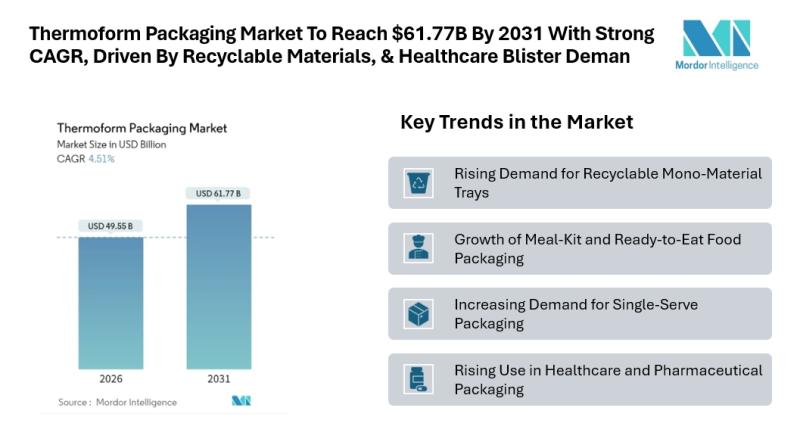
Thermoform Packaging Market to Reach $61.77B By 2031 With A Strong CAGR, Driven …
Mordor Intelligence has published a new report on the thermoform packaging market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Outlook of the Thermoform Packaging Market
According to Mordor Intelligence, the thermoform packaging market size is estimated at USD 49.55 billion in 2026, growing from USD 47.41 billion in 2025 and projected to reach USD 61.77 billion by 2031, registering a CAGR of 4.51% during the forecast period. This…

Florida Physician Launches 2026 "Hardware and Software" Men's Longevity Initiati …
LifeWellMD in Palm Beach and Port St. Lucie, Florida, has launched a 2026 Men's Longevity Initiative that treats the male body like a highperformance system-fixing "hardware" with regenerative and vascular therapies, upgrading "software" with hormone and sleep optimization, and in select cases installing a "new operating system" using advanced cellular options. The program gives men a clear roadmap to improve energy, recovery, focus, and longterm health-span.
Port St. Lucie, FL -…

Tessa Belanger's "PASS THE SAGE" presents 'Stories from the fire, teachings from …
PASS THE SAGE: Stories from the Fire, Teachings from the Smoke is a stirring new anthology from Algonquin author Tessa Belanger that gathers powerful Indigenous voices into one luminous collection. Rooted in ceremony, shaped by community, and told with unflinching honesty. PASS THE SAGE invites readers to sit by the fire, burn our medicines and listen deeply while you carry the teachings forward.
Born from workshops, circles, and lived experience, this…

Prestige Blinds of Coral Springs Named Top Rated Window Treatment Company on Goo …
Prestige Blinds of Coral Springs, a leading provider of custom blinds, shades, and window treatments in Coral Springs, Florida, has earned recognition as a Top Rated Company on Google, based on outstanding customer reviews and five-star ratings.
This achievement reflects the company's reputation as a trusted local window treatment specialist serving Coral Springs and surrounding Broward County areas, including Parkland, Coconut Creek, Margate, Tamarac, and Deerfield Beach. Customers consistently highlight Prestige…
More Releases for Osteosarcoma
Osteosarcoma Drug Market Hits New High | Major Giants AstraZeneca,Pfizer,Amgen,
HTF MI just released the Global Osteosarcoma Drug Market Study, a comprehensive analysis of the market that spans more than 143+ pages and describes the product and industry scope as well as the market prognosis and status for 2025-2032. The marketization process is being accelerated by the market study's segmentation by important regions. The market is currently expanding its reach.
Major companies profiled in Osteosarcoma Drug Market are: Pfizer, Amgen, Bayer,…
Osteosarcoma Market expected to rise | AlaMab Therapeutics, Zentalis Pharmaceuti …
The Osteosarcoma market growth is driven by factors like increase in the prevalence of Osteosarcoma, investments in research and development, entry of emerging therapies during the study period 2019-2032.
The Osteosarcoma market report [https://www.delveinsight.com/report-store/osteosarcoma-market?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr] also offers comprehensive insights into the Osteosarcoma market size, share, Osteosarcoma epidemiology, emerging therapies, market drivers and barriers, ongoing clinical trials, key collaboration in the space, market uptake by key therapies and companies actively pushing Osteosarcoma market…
Exploring the Osteosarcoma Drug Market: Trends, Opportunities, and Challenges
Osteosarcoma, a rare and aggressive bone cancer primarily affecting adolescents and young adults, has garnered significant attention in the pharmaceutical sector due to its complex treatment requirements and evolving drug landscape. This guest post delves into the osteosarcoma drug market, providing insights into its current state, opportunities, challenges, and future trends.
Introduction
Osteosarcoma is the most common primary malignant bone tumor, often occurring in the long bones of the arms and legs.…
Osteosarcoma Market Size, Share, Trends & Forecast - 2034
Overview:
According to the IMARC Group, the osteosarcoma market exhibited a market size of US$ 880.9 Million in the year 2023 and is projected at a CAGR of 4.68% during 2024-2034. This can be attributed to the growing use of combination therapy involving neoadjuvant chemotherapy and surgical resection, which improves treatment outcomes in patients and reduces the risk of cancer relapse.
The osteosarcoma market report offers a comprehensive analysis of the market…
Osteosarcoma Market Report 2023-2033 | Industry Size, Growth and Latest Insights
Market Overview:
The osteosarcoma market is expected to exhibit a CAGR of 4.87% during 2023-2033. The report offers a comprehensive analysis of the osteosarcoma market in the United States, EU5 (including Germany, Spain, Italy, France, and the United Kingdom), and Japan. It covers aspects such as treatment methods, drugs available in the market, drugs in development, the proportion of various therapies, and the market's performance in the seven major regions. Additionally,…
Pediatric Osteosarcoma Therapeutics Market Insights, Forecast to 2031
The report extensively examines the global Pediatric Osteosarcoma Therapeutics market while focusing on the leading companies and their business strategies, geographical growth, market segmentation, competitive environment, production, price, and cost structures. Each section of the research report has been carefully designed to examine important facets of the global market for Pediatric Osteosarcoma Therapeutics. For instance, the market dynamics section delves deeply into the trends, opportunities, and drivers influencing the global…
