Press release
Lentiviral Vectors Market Poised for Significant Growth, Projected to Reach US$ 411.2 Million by 2032 - Persistence Market Research
IntroductionThe lentiviral vectors market is witnessing substantial growth, driven by advancements in gene therapy, increasing research activities, and the rising prevalence of genetic disorders. Lentiviral vectors have emerged as a crucial tool in gene therapy, offering stable and efficient gene delivery into dividing and non-dividing cells. With the growing adoption of these vectors in clinical applications, the market is expected to expand significantly, reaching an estimated valuation of US$ 411.2 million by 2032, according to Persistence Market Research.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/33074
Market Overview
Lentiviral vectors are derived from the lentivirus family, primarily from human immunodeficiency virus type 1 (HIV-1). These vectors are extensively used in gene therapy, immunotherapy, and stem cell research, owing to their high transduction efficiency and ability to integrate genetic material into the host genome. The increasing demand for targeted and personalized therapies has fueled the development of lentiviral vector-based therapeutics, contributing to the market's robust expansion.
Key Market Drivers
Several factors are propelling the growth of the lentiviral vectors market:
1. Rising Prevalence of Genetic Disorders and Chronic Diseases
The increasing incidence of genetic disorders, such as sickle cell anemia, hemophilia, and muscular dystrophy, has necessitated the development of advanced gene therapies. Lentiviral vectors play a vital role in these treatments by facilitating stable gene integration, leading to long-term therapeutic effects. Additionally, chronic diseases such as cancer and HIV/AIDS have further heightened the demand for these vectors in innovative treatment approaches.
2. Expanding Applications in Gene Therapy and Immunotherapy
Lentiviral vectors are extensively used in gene therapy applications for treating inherited genetic disorders, metabolic diseases, and neurodegenerative conditions. The advent of CAR-T cell therapies has further fueled market growth, as these vectors are widely used in engineering T cells to target and eliminate cancer cells. With ongoing advancements in immunotherapy, lentiviral vectors are becoming an integral part of next-generation treatment modalities.
3. Increasing Investments in Biopharmaceutical Research
Biopharmaceutical companies and research institutions are significantly investing in R&D activities related to lentiviral vector-based therapies. The growing focus on developing innovative treatments, coupled with government funding and private sector investments, is driving the expansion of the market. Clinical trials evaluating lentiviral vector applications are increasing, further strengthening the market landscape.
4. Technological Advancements Enhancing Vector Safety and Efficiency
Recent innovations in vector design and manufacturing processes have improved the safety and efficacy of lentiviral vectors. Advanced self-inactivating (SIN) vectors reduce the risk of insertional mutagenesis, enhancing their clinical applicability. Moreover, scalable production techniques and optimized transduction protocols are supporting large-scale commercialization efforts.
Market Challenges
Despite the promising growth prospects, the lentiviral vectors market faces several challenges:
1. High Production Costs and Complex Manufacturing Processes
Lentiviral vector production involves intricate bioprocessing techniques, stringent quality control measures, and expensive raw materials, making it a cost-intensive process. The requirement for specialized infrastructure and skilled professionals further adds to the production costs, limiting accessibility for smaller research institutions and emerging biotech firms.
2. Regulatory Hurdles and Safety Concerns
The stringent regulatory landscape governing gene therapies poses a significant challenge for market players. Regulatory agencies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) impose rigorous approval processes to ensure vector safety and efficacy. Additionally, concerns regarding potential genotoxicity and immune responses require continuous monitoring and advancements in vector engineering.
3. Limited Commercial Availability
Despite extensive research and clinical trials, the commercial availability of lentiviral vector-based therapies remains limited. The long development timelines and high failure rates in clinical trials hinder the market's rapid expansion. Addressing these limitations requires sustained research efforts and collaborative initiatives between academia and industry stakeholders.
Regional Market Insights
1. North America: A Dominant Market Player
North America holds the largest share in the lentiviral vectors market, driven by a strong presence of biopharmaceutical companies, well-established research infrastructure, and favorable regulatory policies. The increasing adoption of gene therapies and the rising incidence of genetic disorders and cancer further contribute to the region's market growth.
2. Europe: Advancements in Gene Therapy Driving Growth
Europe is witnessing significant growth in the lentiviral vectors market, fueled by research advancements and increasing approvals for gene therapy products. Countries such as Germany, the UK, and France are at the forefront of gene therapy research, supporting the expansion of the market.
3. Asia-Pacific: Emerging as a Key Growth Region
The Asia-Pacific region is experiencing rapid market growth due to increasing investments in biotechnology and regenerative medicine. Countries such as China, Japan, and South Korea are actively engaged in gene therapy research, driving demand for lentiviral vectors. Additionally, government initiatives promoting biopharmaceutical research are expected to accelerate market expansion in this region.
Competitive Landscape
The lentiviral vectors market is highly competitive, with key players focusing on strategic collaborations, product innovations, and capacity expansions to gain a competitive edge. Some of the leading companies operating in the market include:
Oxford Biomedica
Thermo Fisher Scientific
SIRION Biotech
Lonza Group
Miltenyi Biotec
Cell Biologics Inc.
Creative Biogene
Vigene Biosciences
These companies are investing in new product launches, licensing agreements, and mergers & acquisitions to strengthen their market presence. For instance, Oxford Biomedica has been at the forefront of lentiviral vector manufacturing, collaborating with leading pharmaceutical companies to advance gene therapy solutions.
Future Market Outlook
The future of the lentiviral vectors market looks promising, with technological innovations, increased clinical adoption, and expanding therapeutic applications driving sustained growth. Key trends expected to shape the market include:
Increased focus on large-scale vector manufacturing to support commercial gene therapy production.
Advancements in gene editing technologies, such as CRISPR-Cas9, enhancing vector performance.
Regulatory advancements aimed at streamlining approval processes for gene therapy products.
Growing partnerships between biotech firms and academic institutions to accelerate research and development.
Conclusion
The lentiviral vectors market is poised for remarkable growth, with a projected valuation of US$ 411.2 million by 2032. The increasing adoption of gene therapy, advancements in immunotherapy, and rising demand for personalized treatments are fueling market expansion. Despite challenges such as high production costs and regulatory complexities, continued investments in R&D and strategic collaborations are expected to drive sustained growth in the sector. As gene therapy continues to revolutionize healthcare, lentiviral vectors will remain a critical component in the development of transformative treatments for a wide range of diseases.
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web:
https://www.persistencemarketresearch.com
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Lentiviral Vectors Market Poised for Significant Growth, Projected to Reach US$ 411.2 Million by 2032 - Persistence Market Research here
News-ID: 3892887 • Views: …
More Releases from Persistence Market Research

Cryogenic Storage Tanks Market Predicted to Hit US$ 12.8 Billion by 2033 Driven …
According to the latest study by Persistence Market Research, the global cryogenic storage tanks market is likely to be valued at US$ 8.6 billion in 2026 and is projected to reach US$ 12.8 billion by 2033, expanding at a CAGR of 5.8% during the forecast period 2026-2033. Rising demand for liquefied gases across energy, healthcare, food processing, and industrial manufacturing sectors is emerging as a key driver shaping the market's…
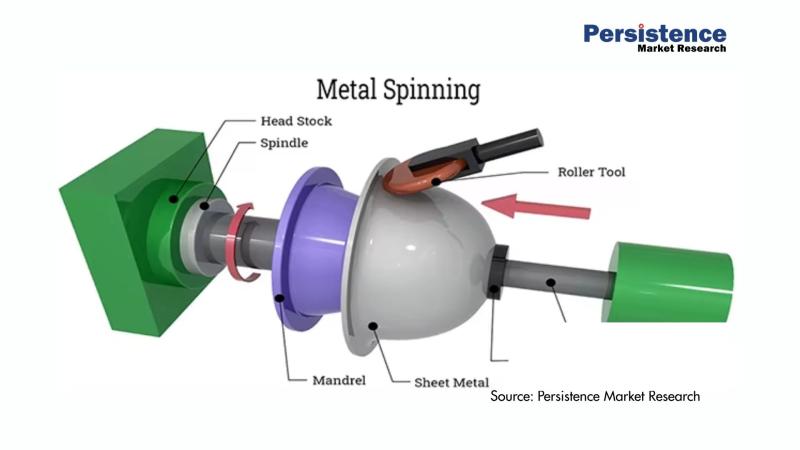
Metal Spinning Products Market Projected to Grow to US$ 4.0 billion by 2033 - Pe …
The global metal spinning products market is poised for substantial growth in the coming years. According to a recent study by Persistence Market Research, the market size is anticipated to reach US$ 4.0 billion by 2033, growing at a robust compound annual growth rate (CAGR) of 4.2% from its current valuation of US$ 3.0 billion in 2026. Metal spinning, a process of shaping metal into precise and symmetrical shapes, is…
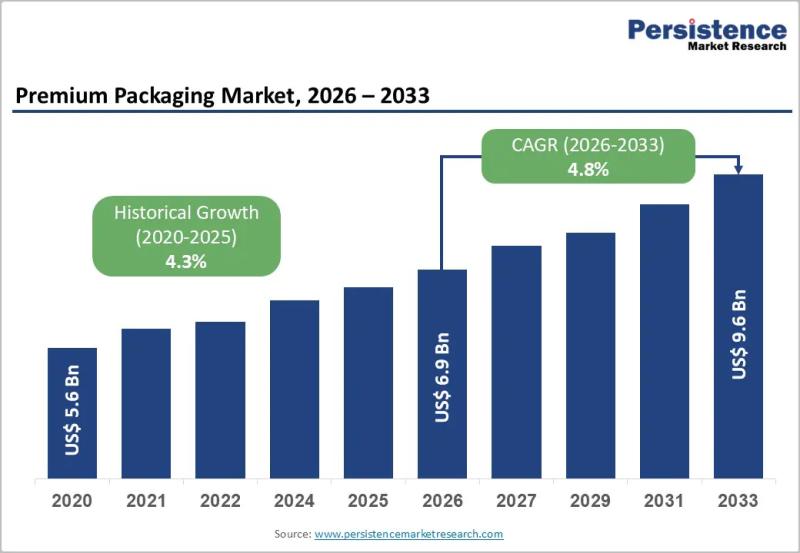
Premium Packaging Market Size Worth US$9.6 Billion by 2033 - Persistence Market …
The premium packaging market has evolved into a critical strategic element for brand differentiation across multiple high value consumer industries. Premium packaging goes beyond basic containment and protection to deliver enhanced aesthetics tactile appeal storytelling and emotional connection. Brands increasingly view packaging as an extension of their identity and a powerful marketing tool that influences purchasing decisions at the point of sale and during the unboxing experience. This shift is…
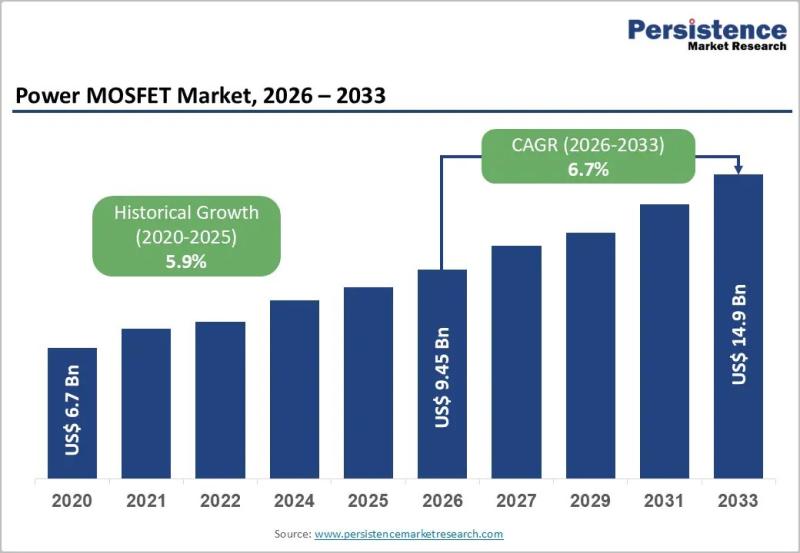
Power MOSFET Market Growth Driven by EVs Renewable Energy and Smart Automation
The global Power MOSFET market is entering a phase of sustained expansion, driven by the accelerating need for energy-efficient and high-performance power management components across industries. In 2026, the market is expected to be valued at US$ 9.45 billion and is forecast to reach US$ 14.9 billion by 2033, registering a healthy CAGR of 6.7% during the forecast period. Power MOSFETs are essential semiconductor devices that enable efficient switching and…
More Releases for Lentiviral
Key Factor Supporting Lentiviral Vector Market Development in 2025: Lentiviral V …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Lentiviral Vector Market Size By 2025?
The size of the lentiviral vector market has expanded significantly in the past few years. Projections show an increase from $14.37 billion in 2024 to $16.48 billion in 2025, reflecting a compound annual growth rate (CAGR) of 14.7%. Factors…
Emerging Lentiviral Vector Market Trend 2025-2034: Technological Advancements In …
How Is the Lentiviral Vector Market Projected to Grow, and What Is Its Market Size?
The lentiviral vector market will grow from $14.37 billion in 2024 to $16.62 billion in 2025, at a CAGR of 15.7%. This market is expanding due to the growing prevalence of genetic disorders, increasing demand for gene delivery systems, biotech and pharmaceutical investments, and regulatory approvals for related therapies.
The lentiviral vector market is expected to grow…
Lentiviral Vector Market Size, Share, and Growth Opportunities 2023 -2030
This Lentiviral Vector Market report has been prepared by considering several fragments of the present and upcoming market scenario. The market insights gained through this market research analysis report facilitates more clear understanding of the market landscape, issues that may interrupt in the future, and ways to position definite brand excellently. It consists of most-detailed market segmentation, thorough analysis of major market players, trends in consumer and supply…
Lentiviral Vectors Market Size 2024 to 2031.
Market Overview and Report Coverage
Lentiviral vectors are a type of viral vector used in gene therapy to deliver genetic material into target cells for both research and therapeutic purposes. These vectors are derived from lentiviruses, a type of retrovirus that can integrate their genetic material into the DNA of host cells.
The Lentiviral Vectors Market is poised for significant growth in the coming years, with a projected CAGR of…
The Lentiviral Vectors Market To Strive With Vertical Saas
Lentiviral vectors are gene delivery mechanisms that are produced from the lentivirus of the human immunodeficiency virus type 1 (HIV-1). These vectors are mainly incompetent for replication, and hence, regarded as generally safe. Yet, they can successfully integrate into the genomic DNA of a wide variety of dividing and non-dividing mammalian cell types.
With recorded sales of US$ 127.6 Mn in 2021, the global lentiviral vectors market is predicted to experience…
Lentiviral Vector Manufacturing Market Size
According to a new market research report published by Global Market Estimates, the Global Lentiviral Vector Manufacturing Market is projected to grow at a CAGR value of 17.8% from 2022 and 2027.
Charles River Laboratories, Vivebiotech, Thermo Fisher Scientific. Inc., GenScript, Creative Biogene, OriGene Technologies, Inc., Oxford Biomedica, Merck KGaA, Takara Bio, Pall Corporation, ITSBio Inc., ANDELYN BIOSCIENCES, Sino Biological Inc., Cellomics Technology, LLC, Virica Biotech, Cell Biolabs, Inc., SignaGen…