Press release
Allogeneic Cell Therapy Market Predicted to Expand to USD 2.4 Billion by 2031 | Persistence Market Research Report
IntroductionAllogeneic cell therapy is rapidly emerging as a transformative approach in regenerative medicine, offering groundbreaking treatments for a range of chronic and life-threatening diseases. Unlike autologous cell therapy, which uses a patient's own cells, allogeneic therapy utilizes cells from a donor, making it a more scalable and efficient option for mass production and commercialization. With the increasing prevalence of chronic diseases, advancements in biotechnology, and a growing interest in regenerative medicine, the allogeneic cell therapy market is witnessing substantial growth.
According to Persistence Market Research, the global allogeneic cell therapy market is projected to reach USD 2.4 billion by 2031, expanding at a CAGR of 24.1%. This exponential growth reflects the increasing investment in research and development, rising adoption of cell-based therapies, and the promising clinical outcomes demonstrated by allogeneic treatments.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/34626
Market Dynamics Driving Growth
Allogeneic cell therapy is gaining traction due to its numerous advantages over traditional treatment modalities. Key factors driving market expansion include advancements in cell engineering, increasing approvals of cell-based therapies, and the growing need for off-the-shelf solutions that can be used immediately without the need for patient-specific customization.
Growing Prevalence of Chronic Diseases and Unmet Medical Needs
The rising incidence of chronic diseases such as cancer, autoimmune disorders, cardiovascular conditions, and neurological disorders is fueling the demand for innovative therapies. Traditional treatment methods often have limitations, including severe side effects and limited long-term efficacy. Allogeneic cell therapy offers a promising alternative by leveraging stem cells and immune-modulating cells to repair damaged tissues, reduce inflammation, and improve patient outcomes.
Advantages Over Autologous Cell Therapy
While autologous cell therapy has been a key player in regenerative medicine, it comes with challenges such as high costs, lengthy preparation times, and variability in patient-specific cell quality. Allogeneic cell therapy, on the other hand, offers several advantages:
Scalability: Cells from a single donor can be used to treat multiple patients, making it more cost-effective and commercially viable.
Immediate Availability: Unlike autologous therapies, which require weeks of processing, allogeneic treatments can be prepared in advance and used when needed.
Standardized Quality: The controlled manufacturing process ensures consistent product quality and therapeutic efficacy.
Increasing Research and Development Activities
Pharmaceutical and biotechnology companies are heavily investing in the development of allogeneic cell therapies. Several clinical trials are underway to explore the potential of allogeneic treatments for various conditions, including graft-versus-host disease (GvHD), hematologic malignancies, musculoskeletal disorders, and autoimmune diseases. The success of these trials is paving the way for regulatory approvals and market expansion.
Technological Advancements in Cell Engineering and Manufacturing
Breakthroughs in gene editing technologies such as CRISPR-Cas9 and TALENs have enhanced the safety and efficacy of allogeneic cell therapies. Scientists are now able to modify donor cells to reduce the risk of immune rejection and increase therapeutic benefits. Additionally, advancements in bioprocessing and automation have improved the scalability and cost-effectiveness of cell therapy production.
Supportive Regulatory Framework and Expedited Approvals
Regulatory agencies such as the FDA and EMA are actively working to streamline the approval process for cell-based therapies. Programs like the Regenerative Medicine Advanced Therapy (RMAT) designation in the U.S. and the Priority Medicines (PRIME) scheme in Europe are providing accelerated pathways for promising therapies. This regulatory support is expected to drive market growth and enable faster commercialization of allogeneic cell therapies.
Strategic Collaborations and Industry Investments
Major pharmaceutical companies, biotech startups, and academic institutions are entering into strategic partnerships to accelerate research and commercialization efforts. Notable collaborations include:
Joint ventures between biotech firms and contract manufacturing organizations (CMOs) to enhance large-scale production capabilities.
Partnerships between pharmaceutical giants and research institutes to advance clinical trials and regulatory approvals.
Mergers and acquisitions aimed at strengthening pipelines and expanding market presence in regenerative medicine.
Key Therapeutic Applications of Allogeneic Cell Therapy
Allogeneic cell therapies are being explored across multiple disease areas, with promising results in several key therapeutic segments.
Oncology and Hematologic Disorders
Cancer treatment is one of the most significant applications of allogeneic cell therapy, particularly in the development of allogeneic CAR-T cell therapies. Unlike traditional CAR-T therapies, which require patient-specific modifications, allogeneic CAR-T cells can be manufactured in bulk and used off-the-shelf. Companies such as Allogene Therapeutics and Cellectis are at the forefront of developing allogeneic CAR-T therapies for leukemia, lymphoma, and multiple myeloma.
Autoimmune Diseases
Allogeneic cell therapy is showing promise in the treatment of autoimmune disorders such as rheumatoid arthritis, lupus, and multiple sclerosis. Mesenchymal stem cells (MSCs) derived from donors have been found to modulate the immune system, reduce inflammation, and promote tissue repair, making them a viable treatment option for autoimmune patients.
Cardiovascular Diseases
Heart failure and ischemic heart disease remain leading causes of morbidity and mortality worldwide. Allogeneic stem cells are being explored for their potential to regenerate damaged cardiac tissues and improve heart function. Studies suggest that allogeneic mesenchymal stem cells (MSCs) and cardiac progenitor cells can enhance myocardial repair and reduce the risk of heart failure progression.
Neurological Disorders
Neurodegenerative diseases such as Alzheimer's, Parkinson's, and spinal cord injuries are challenging to treat with conventional medicine. Allogeneic cell therapy is being investigated as a potential solution to restore neuronal function and slow disease progression. Early-stage trials indicate that donor-derived neural stem cells may help improve cognitive and motor functions in patients suffering from neurological conditions.
Orthopedic and Musculoskeletal Conditions
Allogeneic cell therapy is gaining traction in the treatment of osteoarthritis, tendon injuries, and cartilage defects. Stem cell-based therapies have demonstrated the ability to enhance cartilage regeneration, reduce inflammation, and improve joint function, offering a non-invasive alternative to surgery.
Wound Healing and Tissue Regeneration
Chronic wounds, burns, and diabetic ulcers are significant healthcare burdens. Allogeneic cell therapy has shown promise in promoting faster wound healing and tissue regeneration. Bioengineered skin substitutes containing allogeneic stem cells are already being used in clinical practice to treat severe burns and ulcers.
Challenges and Limitations
Despite its immense potential, the allogeneic cell therapy market faces several challenges:
Immune Rejection and Safety Concerns
One of the biggest hurdles in allogeneic therapy is the risk of immune rejection and graft-versus-host disease (GvHD). While gene editing technologies are addressing these concerns, further research is needed to improve immune tolerance.
High Development and Manufacturing Costs
Producing allogeneic cell therapies is highly complex and requires specialized facilities, stringent quality controls, and expensive raw materials. These costs pose challenges for widespread adoption and accessibility.
Regulatory and Ethical Considerations
Although regulatory agencies are streamlining approval pathways, allogeneic therapies still face rigorous safety and efficacy requirements. Ethical concerns related to donor sourcing and genetic modifications also require careful oversight.
Future Outlook and Market Opportunities
The allogeneic cell therapy market is poised for remarkable growth, driven by technological advancements, increasing regulatory support, and expanding therapeutic applications. Future trends that will shape the industry include:
Integration of Artificial Intelligence (AI) and Machine Learning in Cell Therapy Development to optimize manufacturing and clinical trial efficiency.
Personalized Allogeneic Therapies that combine gene editing with precision medicine to improve patient compatibility and outcomes.
Expansion of Allogeneic CAR-T Therapies Beyond Oncology, targeting infectious diseases and inflammatory disorders.
Development of Universal Donor Cells that can be used across diverse patient populations without the risk of immune rejection.
Conclusion
Allogeneic cell therapy is revolutionizing the field of regenerative medicine, offering new hope for patients suffering from life-threatening and chronic conditions. With an expected market value of USD 2.4 billion by 2031 and a CAGR of 24.1%, the industry is set to witness unprecedented growth.
As research progresses and regulatory frameworks evolve, allogeneic cell therapies have the potential to become mainstream treatment options across multiple disease areas. Overcoming current challenges will require continued innovation, collaboration, and investment, but the future of allogeneic cell therapy is undoubtedly promising, paving the way for a new era of advanced, scalable, and life-changing treatments.
Explore the Latest Trending "Exclusive Article":
· https://medtechpulse.wordpress.com/2025/02/10/surface-disinfectant-market-key-players-and-competitive-landscape-analysis/
· https://medtechpulse.wordpress.com/2025/02/10/ultra-low-temperature-freezer-market-adoption-in-pharma-and-biotech-sectors/
· https://medtechpulse.wordpress.com/2025/02/11/antibody-library-technology-market-driving-precision-medicine-forward/
· https://medium.com/@aishwaryadoiphode15/cell-free-protein-expression-market-advances-in-synthetic-biology-solutions-1206e613b7af
· https://medium.com/@aishwaryadoiphode15/europe-medical-plastic-market-key-trends-shaping-the-industrys-future-growth-881197ff0b2b
· https://www.manchesterprofessionals.co.uk/article/business-management/82523/orthopedic-trauma-devices-market-future-projections-and-investment-insights
· https://www.manchesterprofessionals.co.uk/article/business-management/82534/europe-medical-plastic-market-role-of-recyclable-polymers-in-sustainable-growth
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Allogeneic Cell Therapy Market Predicted to Expand to USD 2.4 Billion by 2031 | Persistence Market Research Report here
News-ID: 3891757 • Views: …
More Releases from Persistence Market Research
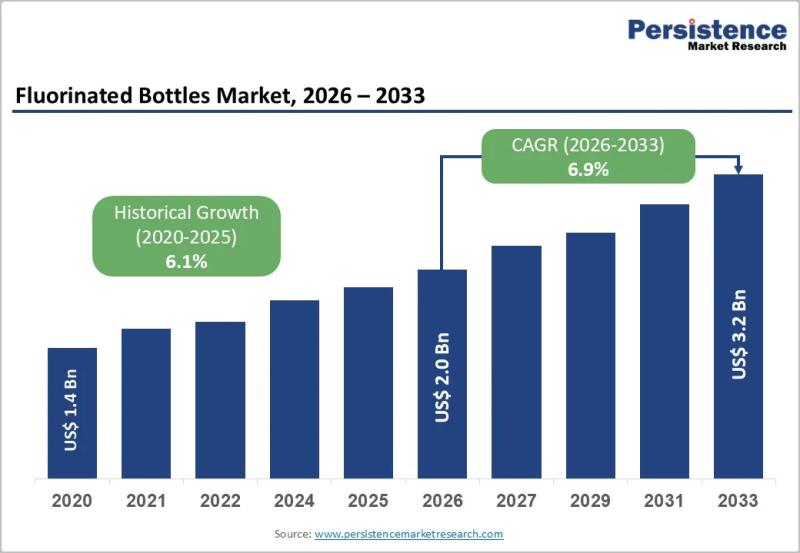
Fluorinated Bottles Market Size Valued at US$ 2.0 Billion in 2026 and Expected t …
The fluorinated bottles market has emerged as a critical segment within the rigid plastic packaging industry, addressing the growing need for safe storage and transportation of aggressive, volatile, and sensitive chemicals. Fluorinated bottles are plastic containers treated through fluorination processes to enhance their barrier properties, chemical resistance, and durability. This treatment significantly reduces permeability and prevents chemical interactions between the container and its contents, making these bottles indispensable for packaging…

Passenger Car Accessories Market to Reach US$ 371.8 Billion by 2032 as Key Playe …
The global Passenger Car Accessories Market is entering a phase of sustained and structurally supported growth, driven by a combination of rising passenger vehicle ownership, shifting consumer preferences toward personalization, and rapid technological advancements across automotive interiors and exteriors. According to consolidated insights from leading market research firms, the market is likely to be valued at US$248.3 billion in 2025 and is projected to reach US$371.8 billion by 2032, expanding…
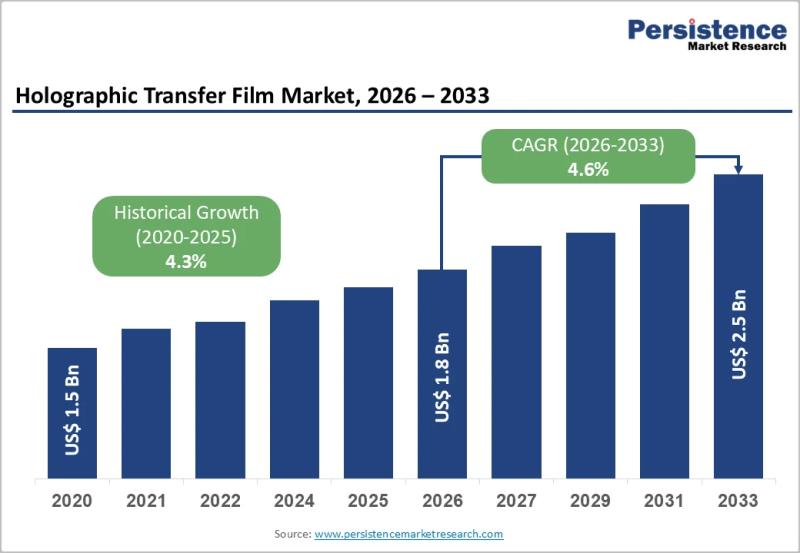
Holographic Transfer Film Market Size Valued at US$1.8 Billion in 2026 and Expec …
The holographic transfer film market is gaining strong momentum as industries increasingly prioritize brand protection visual differentiation and premium packaging aesthetics. Holographic transfer films are widely used to create eye catching metallic and holographic effects on packaging labels cartons textiles and security documents. These films are transferred onto substrates using heat and pressure enabling manufacturers to achieve high visual impact without adding excessive material weight. Their ability to combine decorative…
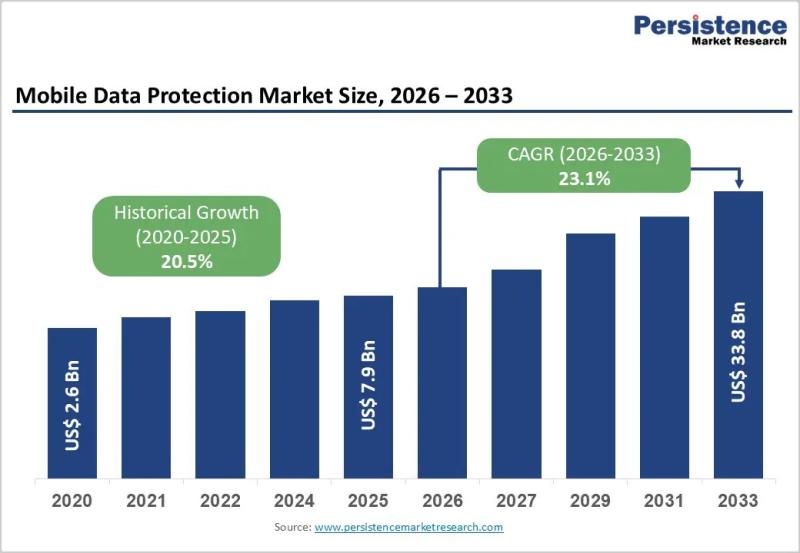
Mobile Data Protection Market Set for Rapid Growth Amid Rising Cyber Threats
Mobile devices have become the backbone of modern digital ecosystems. From enterprise communications and cloud access to mobile payments and healthcare workflows, smartphones and tablets now handle vast volumes of sensitive data. While this shift has dramatically improved productivity and connectivity, it has also exposed organizations to unprecedented cybersecurity risks. As mobile endpoints proliferate across corporate networks-often outside traditional security perimeters-the need for robust mobile data protection has become mission-critical.
The…
More Releases for Allogene
Follicular Lymphoma Pipeline 2025: Therapies Under Investigation, Clinical Trial …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Follicular Lymphoma pipeline constitutes 45+ key companies continuously working towards developing 50+ Follicular Lymphoma treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"Follicular Lymphoma Pipeline Insight, 2025 [https://www.delveinsight.com/sample-request/follicular-lymphoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=gpr]" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Follicular Lymphoma Market.
The Follicular Lymphoma Pipeline report…
Renal Cancer Clinical Pipeline | 75+ Companies, Including Genentech, AstraZeneca …
The Renal Cancer market is evolving with cutting-edge research and new therapeutic advancements.
DelveInsight's 'Renal Cancer Pipeline Insight 2024' report provides comprehensive global coverage of pipeline Renal Cancer therapies in various stages of clinical development. Major pharmaceutical companies are working to advance the pipeline space and future growth potential of the Renal Cancer pipeline domain.
For Renal Cancer emerging drugs, the Renal Cancer pipeline analysis report provides a 360° view of the…
Metastatic Renal Cell Carcinoma Pipeline 2024 | AstraZeneca, Genentech, Sumitomo …
DelveInsight's, "Metastatic Renal Cell Carcinoma Pipeline Insight 2024" report provides comprehensive insights about 40+ companies and 50+ pipeline drugs in Metastatic Renal Cell Carcinoma pipeline landscape. It covers the Metastatic Renal Cell Carcinoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Discover the latest…
Cell Therapy Market 2024 Key Strategies, Trends and Growth Opportunities, Histor …
Market Overview:
Cell therapy involves using cells for therapeutic purpose. It includes various products used for treatment of various chronic diseases like cancer, neurological disorders, cardiovascular diseases.
Market Dynamics:
Rising prevalence of chronic diseases globally is expected to propel the growth of cell therapy market over the forecast period. According to WHO, chronic diseases accounted for over 60% of global deaths in 2016 and cardiovascular diseases were the leading cause of death globally,…
Follicular Lymphoma Pipeline and Clinical Trials Assessment 2024: FDA Approvals, …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Follicular Lymphoma pipeline constitutes 50+ key companies continuously working towards developing 50+ Follicular Lymphoma treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
The Follicular Lymphoma Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed…
Follicular Lymphoma Pipeline Analysis, 2024 Updates | Latest FDA, EMA, and PMDA …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Follicular Lymphoma pipeline constitutes 50+ key companies continuously working towards developing 50+ Follicular Lymphoma treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"Follicular Lymphoma Pipeline Insight, 2024" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Follicular Lymphoma Market.
The Follicular…