Press release
Orphan Drugs Market Size To Hit USD 443.77 Billion by 2030 Amid Rising R&D Investments and Breakthrough Therapies
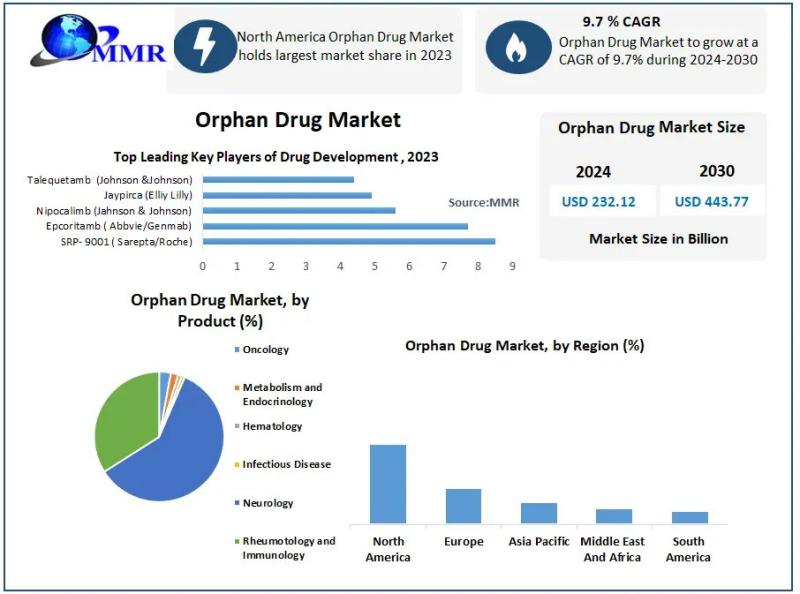
The global orphan drugs market, valued at USD 232.12 billion in 2023, is anticipated to reach approximately USD 443.77 billion by 2030, exhibiting a compound annual growth rate (CAGR) of 9.7% during the forecast period. This growth is primarily driven by technological advancements and favorable regulatory incentives promoting the development of treatments for rare diseases.Request Sample Link For More Details: https://www.maximizemarketresearch.com/request-sample/342/
Market Growth Drivers and Opportunities
Orphan drugs are pharmaceutical agents specifically designed to treat rare medical conditions, often referred to as orphan diseases. The increasing prevalence of rare diseases, coupled with advancements in biotechnology and personalized medicine, has significantly propelled the demand for orphan drugs. Technological innovations such as gene editing, artificial intelligence (AI), and advanced drug delivery systems have revolutionized the development of these specialized medications.
Gene editing technologies, notably CRISPR, have opened new avenues for treating genetic disorders by enabling precise modifications to the human genome. AI and machine learning algorithms facilitate the identification of potential drug candidates and predict their efficacy, thereby accelerating the drug discovery process. Additionally, advanced drug delivery systems enhance the targeted administration of therapies, improving patient outcomes and reducing side effects.
Regulatory frameworks worldwide have established incentives to encourage pharmaceutical companies to invest in orphan drug development. In the United States, the Orphan Drug Act provides benefits such as tax credits, grant funding, and market exclusivity for developers of orphan drugs. Similarly, the European Medicines Agency offers protocol assistance and fee reductions to stimulate research in this domain. These incentives have been instrumental in mitigating the financial risks associated with developing treatments for small patient populations.
Want to access more insights? The journey starts from requesting Sample: https://www.maximizemarketresearch.com/request-sample/342/
Segmentation Analysis
The orphan drugs market is segmented based on product type, disease indication, therapy type, distribution channel, and region.
By Product Type: The market comprises biologic and non-biologic products. Biologics, including gene therapies, monoclonal antibodies, and recombinant proteins, dominate the market due to their efficacy in targeting complex disease mechanisms. The intricate nature of rare diseases often necessitates biologic interventions, which can be tailored to address specific genetic anomalies.
By Disease Indication: Orphan drugs address a wide array of rare conditions, with oncology, hematology, neurology, and metabolic disorders being prominent categories. The oncology segment holds a substantial share, attributed to the high incidence of rare cancers and the continuous development of targeted therapies. Advancements in understanding the molecular underpinnings of these diseases have facilitated the creation of personalized treatment options.
By Therapy Type: The market encompasses various therapeutic approaches, including gene therapy, cell therapy, enzyme replacement therapy, and pharmacological modalities. Gene therapy has gained significant traction, offering potential cures by rectifying underlying genetic defects. Enzyme replacement therapies are particularly effective for metabolic disorders, compensating for deficient or malfunctioning enzymes.
By Distribution Channel: Distribution channels for orphan drugs include hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies are the primary distributors, especially for therapies requiring specialized administration and monitoring. The complexity and specificity of many orphan drugs necessitate controlled dispensing environments to ensure patient safety and therapeutic efficacy.
Country-Level Analysis
United States: The U.S. remains a leader in the orphan drugs market, bolstered by robust healthcare infrastructure, significant investment in research and development, and supportive regulatory policies. The Orphan Drug Act has been pivotal in incentivizing companies to develop treatments for rare diseases, resulting in a substantial pipeline of orphan drugs. Collaborations between academic institutions and pharmaceutical firms further enhance innovation and expedite the availability of new therapies.
Germany: Germany's emphasis on advanced healthcare and medical research has positioned it as a significant market for orphan drugs in Europe. The country's healthcare system supports the adoption of innovative therapies, and regulatory bodies provide pathways for accelerated approval of orphan drugs. Public awareness campaigns and patient advocacy groups in Germany also play a crucial role in promoting early diagnosis and treatment of rare diseases.
China: China is emerging as a key player in the orphan drugs market, driven by a large patient population and increasing healthcare expenditure. Recent regulatory reforms have introduced incentives for orphan drug development, encouraging both domestic and international pharmaceutical companies to invest in this sector. The government's focus on healthcare innovation and the establishment of dedicated funds for rare disease research are expected to further stimulate market growth.
Japan: Japan has a well-established framework for orphan drug designation, offering benefits such as priority review and extended market exclusivity. The country's aging population and high prevalence of certain rare diseases have necessitated the development and approval of orphan drugs. Collaborative efforts between the government, academia, and industry stakeholders facilitate a conducive environment for orphan drug research and commercialization.
Brazil: In Brazil, the orphan drugs market is expanding, supported by government initiatives aimed at improving access to treatments for rare diseases. The National Policy for Rare Diseases outlines strategies for diagnosis, treatment, and research, fostering a supportive ecosystem for orphan drug development. Partnerships between public health institutions and pharmaceutical companies are instrumental in bringing innovative therapies to the Brazilian market.
To Gain More Insights into the Market Analysis, Browse Summary of the Research Report: https://www.maximizemarketresearch.com/market-report/orphan-drugs-market/342/
Competitor Analysis
The orphan drugs market is characterized by the presence of several key players actively engaged in research, development, and commercialization of therapies for rare diseases. Notable companies include:
• Johnson & Johnson: A global healthcare leader, Johnson & Johnson has a diverse portfolio of orphan drugs addressing various rare conditions. The company's commitment to innovation is evident through its substantial investment in research and development, focusing on areas such as oncology and immunology.
• Novartis AG: Novartis is renowned for its extensive pipeline of orphan drugs, particularly in the fields of hematology and neurology. The company's strategic acquisitions and collaborations have bolstered its position in the orphan drugs market, enabling the introduction of cutting-edge therapies.
Pfizer Inc.: Pfizer's portfolio includes several orphan drugs targeting rare diseases, with a strong focus on oncology and gene therapy. The company has actively pursued mergers and acquisitions to strengthen its rare disease treatment capabilities, ensuring a steady pipeline of innovative drugs.
• Bristol-Myers Squibb: A key player in the global orphan drugs market, Bristol-Myers Squibb has been investing heavily in immunotherapies and biologics. The company has consistently expanded its rare disease portfolio through strategic partnerships and acquisitions, reinforcing its position in this specialized sector.
• Sanofi: Sanofi has a well-established presence in the orphan drugs market, particularly in enzyme replacement therapies for metabolic disorders. The company continues to invest in research and development while exploring collaborations to accelerate the delivery of novel treatments for rare diseases.
Beyond these leading companies, several emerging players are making significant strides in orphan drug development, fueled by advancements in biotechnology and regulatory support.
Conclusion
The global orphan drugs market is experiencing remarkable growth, driven by technological advancements, supportive regulatory frameworks, and increasing investments in research and development. With a projected market size of USD 443.77 billion by 2030, the sector is poised for continued expansion, addressing the unmet medical needs of patients with rare diseases.
Biologic therapies, gene editing technologies, and AI-driven drug discovery are transforming the landscape of orphan drug development. Leading pharmaceutical companies, including Johnson & Johnson, Novartis, Pfizer, Bristol-Myers Squibb, and Sanofi, are actively investing in this space, ensuring a robust pipeline of innovative treatments.
As healthcare systems worldwide recognize the significance of orphan drugs, governments and regulatory bodies are implementing policies to facilitate faster approvals and improved access to life-saving therapies. The ongoing advancements in personalized medicine and targeted therapies further strengthen the market's growth potential, making orphan drugs a critical component of the future of healthcare.
For additional reports on related topics, visit our website:
♦Wearable Pregnancy Devices Market https://www.maximizemarketresearch.com/market-report/global-wearable-pregnancy-devices-market/84933/
♦Global Capecitabine Market https://www.maximizemarketresearch.com/market-report/global-capecitabine-market/34503/
♦Latin America Emergency Medical Service (EMS) Products Market https://www.maximizemarketresearch.com/market-report/latin-america-emergency-medical-service-ems-products-market/2880/
♦Incubator Market https://www.maximizemarketresearch.com/market-report/global-incubator-market/95142/
♦Baby & Pregnancy Skincare Products Market https://www.maximizemarketresearch.com/market-report/baby-pregnancy-skincare-products-market/122817/
MAXIMIZE MARKET RESEARCH PVT. LTD.
⮝ 3rd Floor, Navale IT park Phase 2,
Pune Banglore Highway, Narhe,
Pune, Maharashtra 411041, India.
✆ +91 9607365656
🖂 sales@maximizemarketresearch.com
Maximize Market Research is a dynamic market research and consulting firm, bringing together experts from diverse industries. Our expertise spans sectors such as medical devices, pharmaceuticals, science and engineering, electronics, industrial equipment, technology and communication, automotive, chemicals, consumer goods, beverages, personal care, and automation. We offer data-driven industry insights, market forecasts, technical trend analysis, competitive intelligence, strategic consulting, production and demand assessments, and client impact studies, helping businesses navigate evolving market landscapes with confidence.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Orphan Drugs Market Size To Hit USD 443.77 Billion by 2030 Amid Rising R&D Investments and Breakthrough Therapies here
News-ID: 3885745 • Views: …
More Releases from MAXIMIZE MARKET RESEARCH PVT. LTD.

Zero Waste Packaging Market Size, Trends, Growth Drivers & Forecast 2025-2032
Zero Waste Packaging Market was valued at USD 1.26 Bn. in 2024 and is expected to reach USD 1.81 Bn by 2032, at a CAGR of 4.6% during the forecast period.
Zero Waste Packaging Market Overview
The Zero Waste Packaging Market is experiencing rapid growth as businesses and consumers increasingly prioritize sustainability. Zero waste packaging refers to materials and designs that minimize environmental impact by enabling reuse, recycling, composting, or complete biodegradability.…

Industrial Gearbox Market to Reach USD 47.45 Billion by 2032
Industrial Gearbox Market: Growth, Dynamics, and Future Outlook
The Industrial Gearbox Market size was valued at USD 31.40 Billion in 2024 and the total Industrial Gearbox Market revenue is expected to grow at a CAGR of 5.6% from 2025 to 2032, reaching nearly USD 48.60 Billion.
The Industrial Gearbox Market plays a critical role in modern industrial operations by enabling efficient power transmission and speed control across a wide range of applications.…

Pocket Knives Market Outlook 2024-2030: Key Trends and Competitive Landscape
Pocket Knives Market size was valued at USD 625.32 Bn. in 2023 and the total Pocket Knives revenue is expected to grow by 5.7 % from 2024 to 2030, reaching nearly USD 921.78 Bn.
The Pocket Knives Market represents a steadily growing segment within the handheld tools and outdoor equipment industry. Pocket knives are widely used for everyday carry (EDC), outdoor recreation, emergency preparedness, and professional applications. Their compact design, versatility,…

Plastic Extrusion Machine Market: Global Industry Analysis, Size, Growth Trends, …
Plastic Extrusion Machine Market: Growth, Dynamics, and Future Outlook
The Plastic Extrusion Machine Market size was valued at USD 14.20 Billion in 2024, and the total Plastic Extrusion Machine revenue is expected to grow at a CAGR of 6.9% from 2025 to 2032, reaching nearly USD 24.22 Billion.
The Plastic Extrusion Machine Market plays a critical role in the global plastics manufacturing ecosystem. Plastic extrusion machines are widely used to produce pipes,…
More Releases for Orphan
Acquired Orphan Blood Disease Market
Acquired Orphan Blood Disease Market to reach over USD 18.93 billion by the year 2031 - Exclusive Report by InsightAce Analytic
According to a new report by InsightAce Analytic, the "Acquired Orphan Blood Disease Market" in terms of revenue was estimated to be worth $8.65 billion in 2023 and is poised to reach $18.93 billion by 2031, growing at a CAGR of 10.47% from 2024 to 2031.
Get Free Access to…
Orphan Drugs Market Size to Hit $3199.3 Billion by 2028 | Orphan Drugs Industry …
Market Overview:
According to our experience research team, Orphan Drugs Market was valued at USD 112.36 Billion in 2021, and the global Orphan Drugs industry is projected to reach a value of USD 3199.3 Billion by 2028, at a CAGR of 7.4% during the forecast period 2022-2028
Vantage Market Research is a collection of market research studies on several industries, such as Chemicals, semiconductors & Electronics, Food & Beverages Technology, Energy &…
Orphan Drugs for Cancer Pipeline Analysis
A huge market opportunity is offered by small patient population which suffers from rare or orphan diseases. Among the category of new orphan drugs, Oncology account for the largest disease group in recent years. It has been observed that majority of the orphan drugs in the clinical stages are for rare cancer disease drugs, and are in the late stages of the pipeline. Some of the drugs are being developed…
US Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the pharmaceuticals industry.…
Europe Orphan Drugs Pipeline Analysis
“Europe Orphan Drugs Pipeline Analysis” by PNS Pharma gives comprehensive insight on the various drug profiles under Orphan Drugs status in Europe. Research report covers all the ongoing drug development in various phases. Each drug profiles include detailed information like: Originator, Owner, Collaborator, Technology Provider, Licensee, Development Phase, Development Indications, Mechanism of Action, Chemical Formula, Country of Development and detailed analysis on the development process. The information for particular drug…
Global Orphan Drug Pipeline Analysis
In recent years, the pharmaceutical industry has been experiencing a paradigm shift. While a large pool of patients was considered as a major source of revenue for pharma companies in the past, the focus is now gradually shifting to small sections of patients suffering from rare disease. In US & Europe, this pool of patients is gradually growing and orphan drugs are becoming an extremely attractive business proposition for the…