Press release
Europe Biosimilars Market Projected to Hit USD 33.5 Billion by 2031 - Persistence Market Research
IntroductionThe European healthcare landscape is witnessing a transformative shift with the rapid adoption of biosimilars. These biologic drugs, highly similar to already approved reference biologics, offer a cost-effective and equally effective alternative to expensive biologic therapies. The growing acceptance of biosimilars across Europe is driven by increasing healthcare expenditure, patent expirations of blockbuster biologics, and regulatory support from agencies like the European Medicines Agency (EMA).
According to Persistence Market Research, the Europe biosimilars market is projected to reach US$ 33.5 billion by 2031, expanding at a robust CAGR of 15.4% from 2025 to 2031. This significant growth highlights the increasing demand for biosimilars as a key solution to improving patient access to high-quality treatments while reducing the financial burden on healthcare systems.
Get a Sample PDF Brochure of the Report (Use Corporate Email ID for a Quick Response): https://www.persistencemarketresearch.com/samples/34923
Market Dynamics Driving Growth
Rising Demand for Cost-Effective Biologics
Biologic drugs have revolutionized the treatment of chronic diseases such as cancer, autoimmune disorders, and diabetes. However, their high cost often limits patient accessibility. Biosimilars, which provide similar efficacy and safety at a reduced price, are increasingly being adopted as a cost-effective alternative, particularly in European countries with strict healthcare budget constraints.
Patent Expirations of Key Biologics
The expiration of patents for blockbuster biologic drugs, including monoclonal antibodies and growth factors, has opened opportunities for biosimilar manufacturers to enter the market. This has intensified competition and contributed to significant price reductions, fostering wider adoption among healthcare providers and patients.
Regulatory Framework Supporting Biosimilar Approvals
Europe has been at the forefront of biosimilar regulation, with the EMA setting a global benchmark for approval processes. The stringent yet well-defined regulatory framework ensures the safety and efficacy of biosimilars, encouraging manufacturers to develop high-quality products while promoting market growth.
Increasing Investment in Biopharmaceutical Manufacturing
Major pharmaceutical companies and contract manufacturing organizations (CMOs) are expanding their biosimilar production capacities across Europe. With significant investments in state-of-the-art biopharmaceutical manufacturing facilities, the region is becoming a hub for biosimilar production, driving market expansion.
Technological Advancements Enhancing Market Potential
Improved Manufacturing Processes
Advancements in bioprocessing technologies, including cell line engineering, process optimization, and bioreactor innovations, have enhanced the efficiency of biosimilar production. These improvements allow manufacturers to scale up production while maintaining stringent quality standards, reducing overall costs.
Application of Artificial Intelligence in Drug Development
AI-driven predictive analytics and machine learning models are streamlining biosimilar development by optimizing clinical trial designs, accelerating regulatory approvals, and improving pharmacovigilance. These innovations are expediting the time-to-market for biosimilars, fostering increased competition.
Advancements in Analytical Techniques
Innovative analytical methods such as mass spectrometry, chromatography, and bioassays are being employed to ensure biosimilars closely match their reference biologics in terms of structure, purity, and potency. These advancements are strengthening confidence in biosimilar adoption among healthcare providers.
Market Segmentation and Key Therapeutic Applications
By Product Type
The Europe biosimilars market comprises monoclonal antibodies, insulin biosimilars, growth hormones, erythropoietin, and other recombinant proteins. Monoclonal antibodies are the leading segment, driven by their widespread use in oncology, autoimmune diseases, and inflammatory conditions.
By Therapeutic Area
Oncology remains the dominant therapeutic area, with biosimilars playing a crucial role in expanding patient access to life-saving cancer treatments. Autoimmune disorders, including rheumatoid arthritis and inflammatory bowel disease, are also major areas of biosimilar adoption. Additionally, biosimilars for diabetes management and growth hormone deficiencies are gaining significant traction.
By Distribution Channel
Hospital pharmacies, retail pharmacies, and specialty clinics are key distribution channels for biosimilars. Hospital pharmacies dominate the market due to the high demand for biosimilars in oncology and chronic disease management.
Competitive Landscape and Key Players
Leading Biosimilar Manufacturers in Europe
The European biosimilars market is highly competitive, with both global pharmaceutical giants and regional biopharma companies investing in research and development. Key players driving innovation and market expansion include:
Sandoz (Novartis Group) - A pioneer in biosimilars, focusing on expanding its product portfolio and global market presence.
Celltrion Healthcare - Known for its strong pipeline of biosimilars in oncology and autoimmune diseases.
Biogen - Actively involved in the production and commercialization of biosimilars across Europe.
Pfizer - Investing in biosimilar development to enhance patient access to affordable treatments.
Amgen - A leading player in the biosimilar space with a focus on oncology and immunology.
Samsung Bioepis - Gaining market share with its cost-effective biosimilar solutions.
Strategic collaborations, licensing agreements, and mergers and acquisitions are shaping the competitive landscape, enabling companies to expand their product portfolios and strengthen their market presence.
Challenges and Market Barriers
Regulatory Complexity and Market Entry Barriers
Despite Europe's well-established biosimilar regulatory framework, navigating complex approval processes and meeting stringent comparability requirements pose challenges for new entrants. The need for extensive clinical trials and post-marketing surveillance increases development costs.
Physician and Patient Acceptance
Although biosimilars have demonstrated comparable safety and efficacy to reference biologics, physician skepticism and patient concerns about switching from branded biologics remain challenges. Increased education and real-world evidence are essential to improving confidence in biosimilars.
Pricing Pressures and Reimbursement Policies
Price competition among biosimilar manufacturers, coupled with diverse reimbursement policies across European countries, affects market dynamics. Some countries have implemented price caps and tender-based procurement, which can limit profit margins for biosimilar producers.
Future Outlook and Emerging Trends
Expanding Biosimilar Approvals Beyond Oncology
While oncology remains a primary focus, biosimilars for neurology, ophthalmology, and rare diseases are expected to gain traction. Companies are exploring new indications to expand market reach and diversify revenue streams.
Integration of Digital Health Solutions
Digital health technologies, including electronic health records (EHRs) and real-world data analytics, are playing a crucial role in tracking biosimilar utilization, monitoring treatment outcomes, and optimizing patient care. These innovations will further drive biosimilar adoption.
Rise of Interchangeability Designations
The growing trend of interchangeability approvals for biosimilars is expected to boost physician confidence and encourage automatic substitution at the pharmacy level. This will accelerate market penetration and further reduce healthcare costs.
Increasing Partnerships for Market Expansion
Global pharmaceutical companies are forming strategic alliances with regional players to enhance biosimilar commercialization efforts. Partnerships with healthcare providers, payers, and regulatory bodies will play a key role in shaping the future of the biosimilars market.
Conclusion
The Europe biosimilars market is poised for significant expansion, reaching US$ 33.5 billion by 2031, driven by patent expirations, regulatory advancements, and increasing demand for cost-effective biologics. With a CAGR of 15.4% from 2025 to 2031, the market presents lucrative opportunities for pharmaceutical companies, healthcare providers, and policymakers.
Despite challenges such as regulatory hurdles and market access barriers, the long-term outlook remains promising. Continued investments in research, innovative manufacturing techniques, and strategic collaborations will drive biosimilar adoption across therapeutic areas, ultimately transforming the European healthcare landscape. As biosimilars gain wider acceptance, they will play a pivotal role in ensuring sustainable and affordable healthcare solutions for millions of patients across Europe.
Explore the Latest Trending "Exclusive Article":
· https://medtechpulse.wordpress.com/2025/02/10/surface-disinfectant-market-key-players-and-competitive-landscape-analysis/
· https://medtechpulse.wordpress.com/2025/02/10/ultra-low-temperature-freezer-market-adoption-in-pharma-and-biotech-sectors/
· https://medtechpulse.wordpress.com/2025/02/11/antibody-library-technology-market-driving-precision-medicine-forward/
· https://medium.com/@aishwaryadoiphode15/cell-free-protein-expression-market-advances-in-synthetic-biology-solutions-1206e613b7af
· https://medium.com/@aishwaryadoiphode15/europe-medical-plastic-market-key-trends-shaping-the-industrys-future-growth-881197ff0b2b
· https://www.manchesterprofessionals.co.uk/article/business-management/82523/orthopedic-trauma-devices-market-future-projections-and-investment-insights
· https://www.manchesterprofessionals.co.uk/article/business-management/82534/europe-medical-plastic-market-role-of-recyclable-polymers-in-sustainable-growth
About Persistence Market Research:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
Contact Us:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Europe Biosimilars Market Projected to Hit USD 33.5 Billion by 2031 - Persistence Market Research here
News-ID: 3876318 • Views: …
More Releases from Persistence Market Research

Crab Meat Market Value to Reach US$1,691.6 Mn by 2033 Driven by Demand at 5.1% C …
The global crab meat market is poised for stable and sustained growth over the forecast period, driven by increasing consumer demand for premium seafood products, rising health consciousness, and expanding distribution networks. The market is projected to be valued at US$ 1,194.2 million in 2026 and is anticipated to reach approximately US$ 1,691.6 million by 2033, expanding at a compound annual growth rate (CAGR) of 5.1% from 2026 to 2033.…

PDC Drill Bits Market Size to Reach US$ 8.1 Billion by 2033 - Persistence Market …
The polycrystalline diamond compact PDC drill bits market plays a critical role in modern drilling operations across oil and gas mining and geothermal industries. PDC drill bits are engineered using synthetic diamond cutters bonded onto tungsten carbide substrates delivering exceptional hardness wear resistance and cutting efficiency. Compared to traditional roller cone drill bits PDC drill bits offer higher rate of penetration longer service life and reduced downtime making them a…
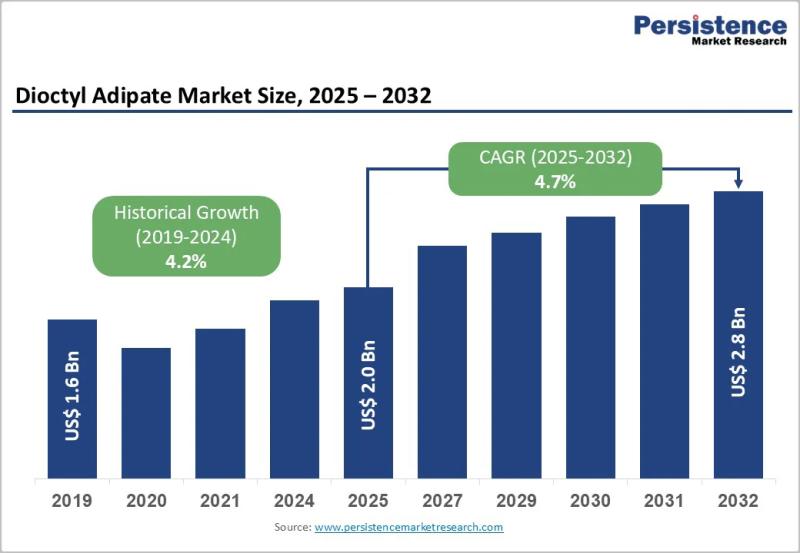
Dioctyl Adipate Market Valued at US$ 2.0 Billion in 2025 Projected to Reach US$ …
The dioctyl adipate market is gaining steady momentum as industries transition toward high performance and flexible plasticizer solutions that meet evolving regulatory and environmental standards. Dioctyl adipate, commonly referred to as DOA, is a widely used plasticizer known for its excellent low temperature flexibility, weather resistance, and compatibility with polyvinyl chloride and other polymers. It plays a critical role in the production of flexible PVC products, synthetic leather, food packaging…

Japan Disposable Cutlery Market to Reach US$103.9 Million by 2033 - Persistence …
The Japan disposable cutlery market represents a niche yet steadily expanding segment within the broader food service packaging industry. Disposable forks, spoons, knives, chopsticks, and hybrid utensil formats play an essential role in Japan's highly organized convenience driven food ecosystem. With one of the world's most advanced retail infrastructures and a strong culture of ready to eat meals, disposable cutlery has become an indispensable component of takeaway, food delivery, and…
More Releases for Europe
2019 Strategy Consulting Market Analysis | McKinsey, The Boston Consulting Group …
Strategy Consulting Market reports also offer important insights which help the industry experts, product managers, CEOs, and business executives to draft their policies on various parameters including expansion, acquisition, and new product launch as well as analyzing and understanding the market trends
Need for strategic planning in highly competitive environment and to develop business capabilities to meet & exceed the emerging requirements are the major drivers which help in surging…
Strategy Consulting Market 2025 | Analysis By Top Key Players: Booz & Co. , Rola …
Global Strategy Consulting Market 2019-2025, has been prepared based on an in-depth market analysis with inputs from industry experts. This report covers the market landscape and its growth prospects over the coming years. The report also includes a discussion of the key vendors operating in this market.
The key players covered in this study
McKinsey , The Boston Consulting Group , Bain & Company , Booz & Co. , Roland Berger Europe…
Digital Strategy Consulting Market is Thriving Worldwide with Deloitte, McKinsey …
A Digital Strategy is a form of strategic management and a business answer or response to a digital question, often best addressed as part of an overall business strategy. A digital strategy is often characterized by the application of new technologies to existing business activity and focus on the enablement of new digital capabilities to their business.
A new report as a Digital Strategy Consulting market that includes a comprehensive analysis…
Strategy Consulting Market 2019: By McKinsey, The Boston Consulting Group, Bain …
This report studies the global Strategy Consulting market, analyzes and researches the Strategy Consulting development status and forecast in United States, EU, Japan, China, India and Southeast Asia. This report focuses on the top players in global market, like
• McKinsey
• The Boston Consulting Group
• Bain & Company
• Booz & Co.
• Roland Berger Europe
• Oliver Wyman Europe
• A.T. Kearney Europe
• Deloitte
• Accenture Europe
Get Sample Report@ https://www.reporthive.com/enquiry.php?id=1247388&req_type=smpl&utm_source=AB
Market segment by Type, the product can be split into
• Operations Consultants
• Business Strategy Consultants
• Investment Consultants
• Sales and…
Strategy Consulting Market Analysis 2018: McKinsey, The Boston Consulting Group, …
Orbis Research Present’s “Global Strategy Consulting Market” magnify the decision making potentiality and helps to create an effective counter strategies to gain competitive advantage.
The global Strategy Consulting status, future forecast, growth opportunity, key market and key players. The study objectives are to present the Strategy Consulting development in United States, Europe and China.
In 2017, the global Strategy Consulting market size was million US$ and it is expected to reach million…
Influenza Vaccination Market Global Forecast 2018-25 Estimated with Top Key Play …
UpMarketResearch published an exclusive report on “Influenza Vaccination market” delivering key insights and providing a competitive advantage to clients through a detailed report. The report contains 115 pages which highly exhibits on current market analysis scenario, upcoming as well as future opportunities, revenue growth, pricing and profitability. This report focuses on the Influenza Vaccination market, especially in North America, Europe and Asia-Pacific, South America, Middle East and Africa. This…
