Press release
Major Market Shift in Cardiovascular Clinical Trials Industry: Cereno Scientific Partners With CRO For Phase I Study
What Is the Forecasted Market Size and Growth Rate for the Cardiovascular Clinical Trials Market?In the past few years, the cardiovascular clinical trials industry has seen considerable growth. The market, which was $5.34 billion in 2024, is projected to expand to $5.73 billion in 2025, experiencing a compound annual growth rate (CAGR) of 7.4%. This substantial growth during the historical period is due to several factors, including an upsurge in myocardial infarctions, regulatory shifts, a heightened disease burden, progress in the field of genomics, and an increased need for new drug development.
There is an anticipated robust expansion in the cardiovascular clinical trials market in the upcoming years. It is projected to surge to $7.53 billion by 2029, with a compound annual growth rate (CAGR) of 7.0%. The expected growth during the forecasted period can be linked to factors such as an aging demographic, emerging markets, the rise of precision medicine, advanced digital health technologies, and an emphasis on patient-oriented trials. Important trends for the upcoming period are expected to be greater collaborations, advancements in technology, product innovation, introduction of new products, and the release of generic versions of combination drugs.
What Is Stimulating Growth in the Cardiovascular Clinical Trials Market?
The increase in cases of heart diseases is projected to boost the advancement of the cardiovascular clinical trials market. Cardiovascular disease pertains to a variety of conditions that affect the heart and blood vessels, which include coronary artery disease, heart failure, and stroke. The surge in cardiovascular diseases can be attributed to numerous factors such as inactive lifestyles, poor diets, smoking, obesity, hypertension, diabetes, and genetic factors. Cardiovascular clinical trials are crucial in identifying and confirming new medications that can more effectively control risks like high blood pressure, elevated cholesterol, and diabetes, which play a significant role in cardiovascular diseases. For example, in September 2024, it was reported by the Minnesota Department of Health, an American state health organization, that about 30% of adults in Minnesota, which is approximately 1.4 million people, had high blood pressure in 2023, and hypertensive diseases accounted for nearly 28% of all deaths in the state, totaling 14,225 residents in 2022. Thus, the rising prevalence of cardiovascular diseases is fueling the progression of the cardiovascular clinical trials market.
Get Your Free Sample Now - Explore Exclusive Market Insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=15532&type=smp
Which Businesses Are at the Forefront of Cardiovascular Clinical Trials Market Development?
Major companies operating in the cardiovascular clinical trials market are Pfizer Inc., Johnson & Johnson, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., AstraZeneca PLC, Novartis AG, Eli Lilly and Company, Gilead Sciences Inc., Amgen Inc., Boehringer Ingelheim International GmbH, Merck & Co. Inc., Baxter International Inc., IQVIA Holdings Inc., SGS S.A., PPD Inc., WuXi AppTec Co. Ltd., Caidya, Syneos Health Inc., Charles River Laboratories International Inc., Sanofi, ICON plc, Medpace Holdings Inc., Cardiovascular Clinical Sciences., ProRelix Services LLP, Worldwide Clinical Trials
What Are the Latest Innovations in the Cardiovascular Clinical Trials Market?
Key players in the cardiovascular clinical trials market are strategically forming partnerships, like those with contract research organizations (CRO), to broaden their distribution networks and appeal to a more extensive customer base. CRO alliances are vital in the clinical research sector, involving cooperative efforts between various companies to deliver specialist services and backing for many elements of clinical trials and research. An example of this is when Cereno Scientific AB, a biopharmaceutical organization in Sweden, joined forces with Clinical Trial Consultants (CTC), a Swedish full-service CRO focusing on clinical proceedings, in September 2023. They collaborated to undertake a Phase I investigation for CS014, an inhibitor of histone deacetylase with an objective to prevent arterial and venous thrombosis. CTC is also contributing to Phase I preparation tasks such as study protocol formulation and the clinical trial's application process, which will take place in Sweden. This first test on humans in Phase I is set to start in the initial part of 2024. This cooperative endeavor marks a significant progression in cardiovascular health research.
How Is the Cardiovascular Clinical Trials Market Segmented?
The cardiovascular clinical trials market covered in this report is segmented -
1) By Phase: Phase I, Phase II, Phase III, Phase IV
2) By Study Design: Interventional, Observational, Expanded Access
3) By Indication: Acute Coronary Syndrome, Coronary Artery Disease, Ischemic Heart Disease, Pulmonary Arterial Hypertension, Stroke, Cardiac Arrhythmias, Heart Failure, Other Indications
Subsegments:
1) By Phase I: First-in-Human Trials, Dose Escalation Studies, Safety and Tolerability Studies, Pharmacokinetics And Pharmacodynamics Studies
2) By Phase II: Efficacy Studies, Optimal Dosage And Administration Route Studies, Early Safety And Efficacy Trials, Biomarker Development Trials
3) By Phase III: Large-Scale Efficacy Trials, Randomized Controlled Trials (RCTs), Long-Term Safety And Efficacy Studies, Multicenter Trials
4) By Phase IV: Post-Marketing Surveillance, Long-Term Safety Studies, Real-World Evidence (RWE) Studies, Comparative Effectiveness Research
Pre-Book Your Report Now For A Swift Delivery:
https://www.thebusinessresearchcompany.com/report/cardiovascular-clinical-trials-global-market-report
Where Is the Cardiovascular Clinical Trials Market Growth Most Prominent?
North America was the largest region in the cardiovascular clinical trials market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the cardiovascular clinical trials market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Is Covered In The Cardiovascular Clinical Trials Global Market Report?
- Market Size Analysis: Analyze the Cardiovascular Clinical Trials Market size by key regions, countries, product types, and applications.
- Market Segmentation Analysis: Identify various subsegments within the Cardiovascular Clinical Trials Market for effective categorization.
- Key Player Focus: Focus on key players to define their market value, share, and competitive landscape.
- Growth Trends Analysis: Examine individual growth trends and prospects in the Market.
- Market Contribution: Evaluate contributions of different segments to the overall Cardiovascular Clinical Trials Market growth.
- Growth Drivers: Detail key factors influencing market growth, including opportunities and drivers.
- Industry Challenges: Analyze challenges and risks affecting the Cardiovascular Clinical Trials Market.
- Competitive Developments: Analyze competitive developments, such as expansions, agreements, and new product launches in the market.
Unlock Exclusive Market Insights - Purchase Your Research Report Now!
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=15532
Connect with us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company,
Twitter: https://twitter.com/tbrc_info,
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ.
Contact Us
Europe: +44 207 1930 708,
Asia: +91 88972 63534,
Americas: +1 315 623 0293 or
Email: mailto:info@tbrc.info
Learn More About The Business Research Company
With over 15,000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Our flagship product, the Global Market Model delivers comprehensive and updated forecasts to support informed decision-making.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Major Market Shift in Cardiovascular Clinical Trials Industry: Cereno Scientific Partners With CRO For Phase I Study here
News-ID: 3859178 • Views: …
More Releases from The Business Research Company
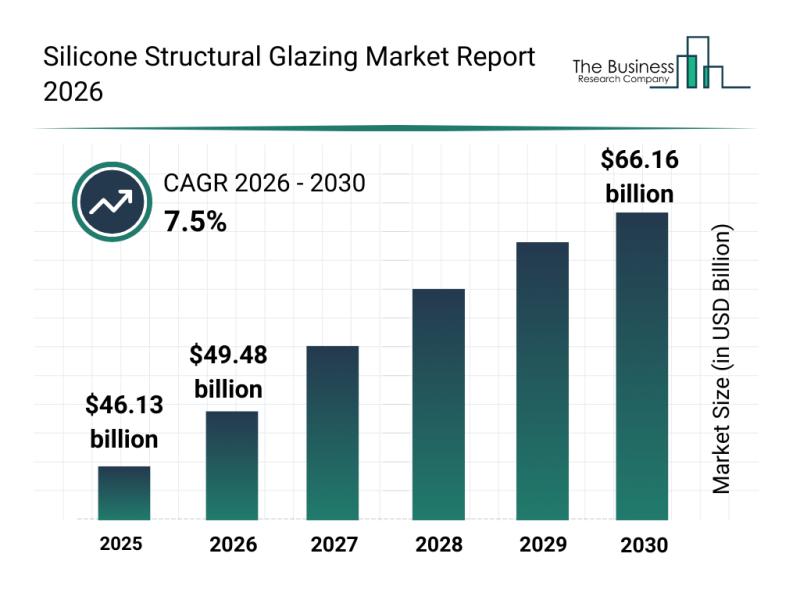
Leading Companies Solidify Their Presence in the Silicone Structural Glazing Mar …
The silicone structural glazing market is positioned for significant expansion in the coming years, driven by advances in building technology and increased environmental awareness. This sector is evolving rapidly as demand grows for more energy-efficient and aesthetically appealing architectural solutions. Let's explore the market's current size, key players, emerging trends, and the main segments that are shaping its future.
Silicone Structural Glazing Market Value Forecast Through 2030
The market for silicone…
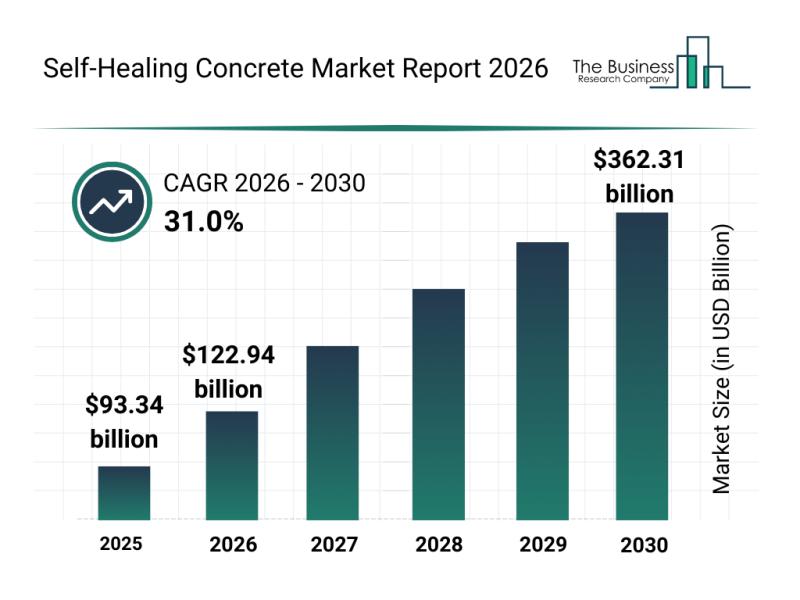
Future Prospects: Key Trends Shaping the Self-Healing Concrete Market up to 2030
The self-healing concrete market is capturing significant attention as innovations and sustainability demands rise in construction. This sector is set to experience remarkable growth due to advancements in materials and technology, shaping the future of durable and intelligent infrastructure solutions. Let's explore the market's size, key players, emerging trends, and segment outlook to understand its trajectory.
Projected Market Size and Growth Prospects for the Self-Healing Concrete Market
The self-healing concrete market…
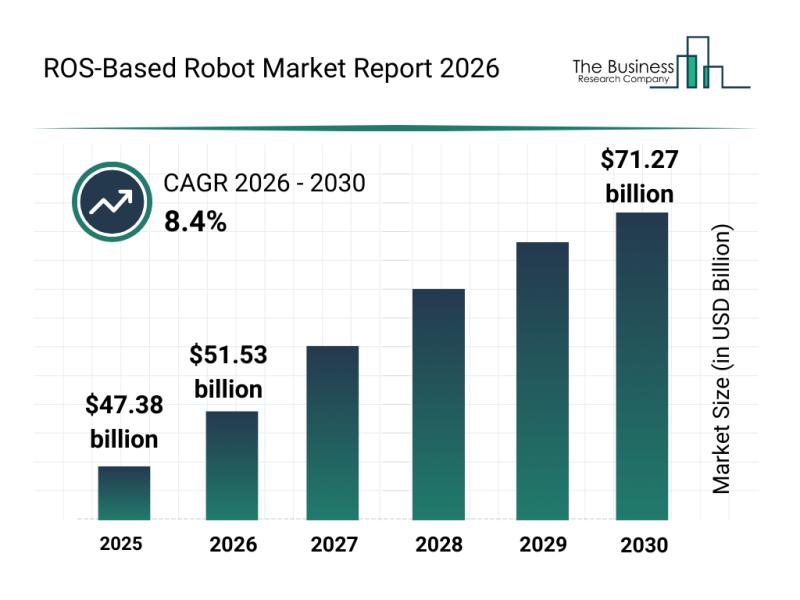
Analysis of Key Market Segments Driving the ROS-Based Robot Industry
The ROS-based robot market is positioned for substantial growth as robotics technology continues to advance rapidly. With increasing innovation in software, hardware, and AI integration, this sector is set to transform multiple industries by 2030. Below, we explore the market's future size, leading companies, key trends, and segmentation details to understand its evolving landscape.
Projected Market Size and Expansion of the ROS-Based Robot Market
The ROS-based robot market is anticipated to…
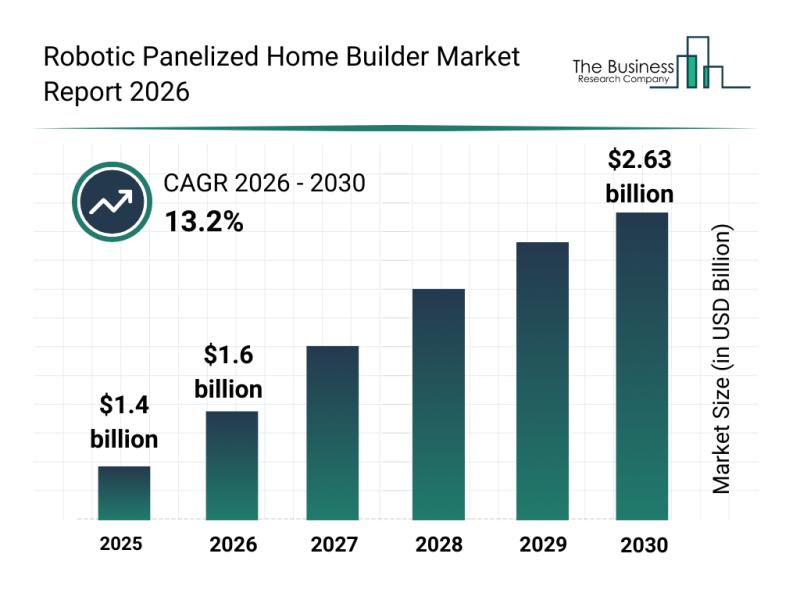
Global Trends Overview: The Rapid Evolution of the Robotic Panelized Home Builde …
The robotic panelized home builder market is positioned for impressive growth in the coming years as automation and robotics increasingly transform construction processes. Driven by technological advancements and expanding prefab housing projects, this market is set to reshape how homes are built with greater speed and efficiency. Let's explore the market's size, leading companies, emerging trends, and key segments that are shaping its future.
Strong Growth Forecast for the Robotic Panelized…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…
