Press release
Spasticity Clinical Trials and Studies 2025: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies by DelveInsight
DelveInsight's, "Spasticity Pipeline Insight" report provides comprehensive insights about 20+ companies and 20+ pipeline drugs in Spasticity pipeline landscape. It covers the Spasticity pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Spasticity therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Stay ahead with the latest insights! Download DelveInsight's comprehensive Spasticity Pipeline Report to explore emerging therapies, key Spasticity Companies, and future Spasticity treatment landscapes @ Spasticity Pipeline Outlook Report [https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Spasticity Pipeline Report
* In January 2025:- Ipsen :- The purpose of the study is to assess the safety and efficacy of increasing doses of IPN10200 with the aim to evaluate the Pharmacodynamics (PD) profile of IPN10200 and to establish the total IPN10200 doses(s) that offer the best efficacy/safety profile when used for the treatment of Adult upper limb (AUL) spasticity.
* In January 2025:- Chongqing Claruvis Pharmaceutical Co., Ltd.:- This is a randomized, double-blind, dose escalation, placebo- and active-controlled parallel-group multi-center phase II study to evaluate the efficacy and safety of Recombinant Botulinum Toxin Type A (YY001) for injection in the treatment of upper limb spasticity in adults.
* In January 2025:- Pacira Pharmaceuticals Inc.:- This multicenter, randomized, double-blind, sham-controlled study is designed to evaluate the efficacy and safety of the iovera degrees system in subjects with upper extremity spasticity. A total of approximately 132 subjects will be enrolled; 88 subjects will receive treatment with the iovera degrees system and 44 subjects will receive sham treatment (sham iovera degrees system treatment).
* DelveInsight's Spasticity pipeline report depicts a robust space with 20+ active players working to develop 20+ pipeline therapies for Spasticity treatment.
* The leading Spasticity Companies such as RVL Pharmaceuticals, Huons, Bionorica SE, Supernus Pharmaceuticals, Saol Therapeutics, Revance Therapeutics, Flex Pharma, Canbex Therapeutics, Ipsen, Lundbeck A/S, CKD Bio Corporation, Sentynl Therapeutics, Shionogi, Lexicon Pharmaceuticals, Incannex, Delpor, Ethicann Pharmaceuticals and others.
* Promising Spasticity Therapies such as IPN10200, SL-1002, SPARC0921, Tizanidine, Moxifloxacin and others.
Discover how the Spasticity treatment paradigm is evolving. Access DelveInsight's in-depth Spasticity Pipeline Analysis for a closer look at promising breakthroughs @ Spasticity Clinical Trials and Studies [https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Spasticity Emerging Drugs Profile
* HU-014: Huons
HU-014 (Liztox) is a newly developed Botulinum toxin A. Botulinum toxin dose is measured in units (U) based on the median lethal dose (LD50) of the neurotoxin. HU-014 is a highly purified and clinically tested injectable neuromodulator with a 900 kDa protein complex derived from neurotoxins produced by Clostridium botulinum, the bacterium responsible for botulism. The botulinum toxin weakens or paralyzes skeletal muscle by inhibiting neurotransmission between peripheral nerve endings and muscle fibers. The effects of botulinum toxin are transient; muscular function typically returns to baseline within a few months.
* Myobloc: Supernus Pharmaceuticals
Myobloc (rimabotulinumtoxinB) injection is the first and only approved botulinum toxin Type B injectable indicated for the treatment of cervical dystonia to reduce the severity of abnormal head position and neck pain associated with cervical dystonia in adults and the treatment of chronic sialorrhea in adults. MYOBLOC blocks cholinergic transmission at the neuromuscular and salivary neuroglandular junction by inhibiting the release of acetylcholine from peripheral cholinergic-nerved terminals. Currently it is being evaluated in Phase II/III clinical trials to treat upper and lower limb spasticity.
Get a detailed analysis of the latest innovations in the Spasticity pipeline. Explore DelveInsight's expert-driven report today! @ Spasticity Unmet Needs [https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Spasticity Companies
RVL Pharmaceuticals, Huons, Bionorica SE, Supernus Pharmaceuticals, Saol Therapeutics, Revance Therapeutics, Flex Pharma, Canbex Therapeutics, Ipsen, Lundbeck A/S, CKD Bio Corporation, Sentynl Therapeutics, Shionogi, Lexicon Pharmaceuticals, Incannex, Delpor, Ethicann Pharmaceuticals and others.
Spasticity pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Oral
* Parenteral
* Intravenous
* Subcutaneous
* Topical
Spasticity Products have been categorized under various Molecule types such as
* Monoclonal Antibody
* Peptides
* Polymer
* Small molecule
* Gene therapy
Download DelveInsight's latest report to gain strategic insights into upcoming therapies and key developments @ Spasticity Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Spasticity Pipeline Report
* Coverage- Global
* Spasticity Companies- RVL Pharmaceuticals, Huons, Bionorica SE, Supernus Pharmaceuticals, Saol Therapeutics, Revance Therapeutics, Flex Pharma, Canbex Therapeutics, Ipsen, Lundbeck A/S, CKD Bio Corporation, Sentynl Therapeutics, Shionogi, Lexicon Pharmaceuticals, Incannex, Delpor, Ethicann Pharmaceuticals and others.
* Spasticity Therapies - IPN10200, SL-1002, SPARC0921, Tizanidine, Moxifloxacin and others.
* Spasticity Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Spasticity Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Which companies are leading the race in Spasticity drug development? Find out in DelveInsight's exclusive Spasticity Pipeline Report-access it now! @ Spasticity Emerging Drugs and Major Companies [https://www.delveinsight.com/sample-request/spasticity-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Spasticity Overview
* Spasticity Pipeline Therapeutics
* Spasticity Therapeutic Assessment
* Spasticity - DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* HU-014: Huons
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II/III)
* Myobloc: Supernus Pharmaceuticals
* Drug profiles in the detailed report.....
* Mid- Stage Products (Phase II)
* LX-9211: Lexicon Pharmaceuticals
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* Lu AG06466: Lundbeck A/S
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Drug profiles in the detailed report.....
* Inactive Products
* Spasticity Key Companies
* Spasticity Key Products
* Spasticity Unmet Needs
* Spasticity Market Drivers
* Spasticity Market Barriers
* Spasticity Future Perspectives and Conclusion
* Spasticity Analyst Views
* Spasticity Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=spasticity-clinical-trials-and-studies-2025-ema-pdma-fda-approvals-mechanism-of-action-roa-nda-ind-and-companies-by-delveinsight]
Phone: 9650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/report-store/knee-reconstruction-devices-market
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Spasticity Clinical Trials and Studies 2025: EMA, PDMA, FDA Approvals, Mechanism of Action, ROA, NDA, IND, and Companies by DelveInsight here
News-ID: 3855021 • Views: …
More Releases from ABNewswire

Elevator Door Operation Debugger - Industry Trends and Best Practices
Elevator door motor inverter controller NSFC01-02 operation debugger [https://www.thoyelevator.com/products/]
Image: https://www.thoyelevator.com/uploads/WPS.png
An elevator door operation debugger, often called a service tool, operator debugger, or handheld server, is a specialized diagnostic device used by technicians to configure, test, and troubleshoot elevator door controllers.
Core Functions-Modern door systems use closed-loop controllers that require specific parameters for safe operation.
Debuggers allow technicians to:
1Self-Learning: Calibrate door width and travel limits by initiating a "learning" cycle where the door…

McKay Law Opens New Greenville Office, Expanding Access to Elite Traumatic Brain …
McKay Law PLLC, East Texas's premier personal injury law firm, opens new Greenville, TX office to serve accident victims throughout Hunt County with specialized expertise in traumatic brain injury (TBI), catastrophic spine, neck, and back injury cases. Located at 2920 Lee St, Ste. 201 Greenville, Texas 75401, McKay Law's new office provides easily accessible, expert legal representation to Greenville residents and surrounding communities who have suffered life-altering injuries
GREENVILLE, TX -…

What effect does diosmin have on the brain?
The following is a detailed breakdown:
Effects of Diosmin [https://www.diosminsupplier.com/protection-of-blood-vessels-diosmin-product/] on the Brain
Antioxidant and Neuroprotective Effects
Diosmin has potent antioxidant properties, neutralizing free radicals and reducing oxidative stress-induced damage to nerve cells. Oxidative stress is a key pathological mechanism in many neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease. By reducing oxidative stress, diosmin may help slow the progression of these diseases.
For example, in rodent models, diosmin attenuated neuronal damage under…
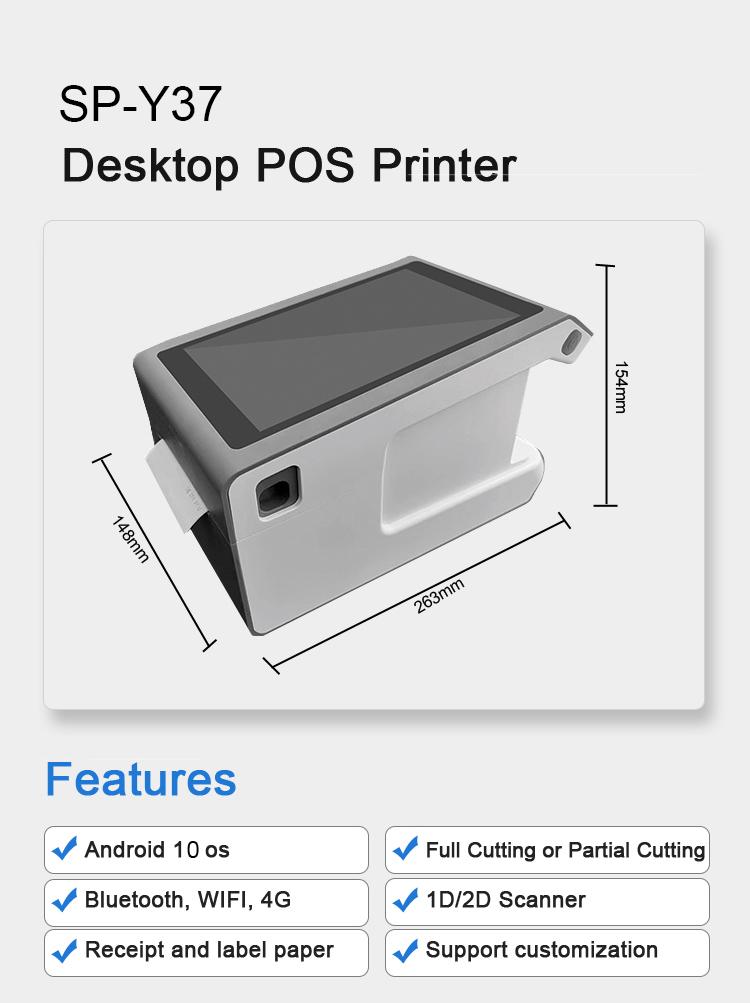
SPRT Y37 Android 8-inch Vertical LCD Screen Printer
SPRT Android terminal printer Y37 [https://www.sprt-printer.com/sprt-y37-android-8-inch-vertical-lcd-screen-printer/] has superior performance.
1D and 2D code can be scanned. It also supports voice broadcasting.
The data of each receipt can be converted into text and uploaded to the cloud, which facilitates the collection and management of big data.
It is mostly used in the takeaway industry, distribution industry, bill printing, electronic cash register POS system bill printing, sports/postal/civil aviation bill printing, service system bill printing, tax/billing…
More Releases for Spasticity
Spasticity Market: Rapid Growth & Investment Outlook to 2032 - DelveInsight
The Spasticity market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Spasticity pipeline products will significantly revolutionize the Spasticity market dynamics.
DelveInsight's "Spasticity Market Insights, Epidemiology, and Market Forecast-2032′′ report offers an in-depth understanding of the Spasticity, historical and forecasted epidemiology as well as the Spasticity market trends in the United States, EU5 (Germany, Spain, Italy, France,…
Spasticity Market 2025-2034 Business Outlook, Critical Insight and Growth
Introduction
Spasticity is a motor disorder characterized by involuntary muscle stiffness, spasms, and exaggerated reflexes caused by damage to the central nervous system (CNS). It frequently occurs as a complication of stroke, multiple sclerosis (MS), cerebral palsy, traumatic brain or spinal cord injury, and other neurological conditions. Spasticity can severely impact mobility, daily functioning, and quality of life, making it a significant healthcare burden worldwide.
Treatment approaches combine pharmacological agents, intrathecal therapies,…
Muscle Spasticity Market Emerging Trends and Growth Prospects 2034
Introduction
Muscle spasticity is a condition characterized by involuntary muscle stiffness, spasms, and increased tone, often resulting from neurological disorders such as multiple sclerosis, cerebral palsy, stroke, or traumatic brain and spinal cord injuries. It significantly impacts mobility and quality of life, making effective management essential. With the rising incidence of neurological diseases and advancements in physiotherapy, drug therapies, and medical devices, the global muscle spasticity market is witnessing steady expansion.
According…
Spasticity Drugs Market Segments, Growth and Trends by Forecast by 2031
The report is segmented By Drug Type (Baclofen, Botulinum Toxin, Diazepam, Tizanidine, Dantrolene Sodium, and Others), Route of Administration (Oral, Intramuscular, and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Get Sample Copy -https://www.theinsightpartners.com/sample/TIPRE00040811/?utm_source=OpenPR&utm_medium=10831
Major Companies operating in the Spasticity Drugs Market are:
Ipsen Pharma
Allergan
Acorda Therapeutics,…
Spasticity Drugs Top Companies Regional and Data Analysis to 2031
The Spasticity Drugs Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits.
Download PDF Brochure at: https://www.theinsightpartners.com/sample/TIPRE00040811/?utm_source=OpenPR&utm_medium=10129
Major Companies operating in the Spasticity Drugs Market are:
• Ipsen Pharma
• Allergan
• Acorda Therapeutics, Inc.
• Merz Pharma
• Teva Pharmaceutical Industries Ltd.
• Novartis AG
• Sun Pharmaceutical Industries Ltd.
• Beximco Pharmaceuticals Ltd.
• Johnson & Johnson Private Limited
• Zydus Cadila
Contact Us:
If you have any queries about this report or if…
Spasticity Treatment Market Expecting Huge Demand in Upcoming Years
The Global Spasticity Treatment Market size is estimated at $5.7 Billion in 2025 and is forecast to register an annual growth rate (CAGR) of 4.9% to reach $8.8 Billion by 2034. Download now
The latest study released on the Global Spasticity Treatment Market by USD Analytics Market evaluates market size, trend, and forecast to 2034. The Spasticity Treatment market study covers significant research data and proofs to be a handy resource…
