Press release
Clinical Trial Management System Market: Company Profiles, Segment Size, and Forecast Through 2024-2031 | Oracle, IQVIA Inc, BioClinica.
The Global Clinical Trial Management System Market reached USD 1.5 billion in 2022 and is projected to witness lucrative growth by reaching up to USD 4.2 billion by 2031. The global clinical trial management system market is expected to exhibit a CAGR of 14.6% during the forecast period (2024-2031).The Clinical Trial Management System market report provides in-depth insights and analysis on key market trends, growth opportunities, and emerging challenges. With a commitment to delivering actionable intelligence, DataM Intelligence empowers businesses to make informed decisions and stay ahead of the competition. Leveraging a combination of qualitative and quantitative research methods, the company offers comprehensive reports that help clients navigate complex market landscapes, drive strategic growth, and seize new opportunities in an ever-evolving global market.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://datamintelligence.com/download-sample/clinical-trial-management-system-market?sz
The Clinical Trial Management System (CTMS) Market focuses on software solutions that streamline clinical trial planning, tracking, and management. Driven by the rise in clinical research, regulatory compliance needs, and digital transformation in healthcare, CTMS enhances trial efficiency, data management, and patient recruitment. The market is expanding with cloud-based solutions and AI-driven analytics for improved decision-making.
Forecast Growth Projected:
The Global Clinical Trial Management System Market is anticipated to rise at a considerable rate during the forecast period, between 2024 and 2031. In 2023, the market is growing at a steady rate, and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
Transform your pharma strategy with expert insights and competitive analysis: https://www.datamintelligence.com/competitive-intelligence/contact
List of the Key Players in the Clinical Trial Management System Market:
Oracle, IQVIA Inc., RealTime Software Solutions, LLC, Bio-Optronics, Medidata (Dassault Systèmes), BioClinica, Clario, Forte Research Systems, SimpleTrials, and Calyx, among others.
Industry Development:
On April 21, 2022, Bristol Myers Squibb adopted Veeva Vault CTMS globally to enhance end-to-end clinical trial management, as announced by Veeva Systems. This advanced CTMS replaced older systems from both Celgene and Bristol Myers Squibb, seamlessly integrating with Veeva's eTMF and Study Startup solutions. The transition was completed in under 20 months.
On August 17, 2021, Verily moved to acquire SignalPath, a North Carolina-based software company, as part of its efforts to expand Project Baseline, its clinical research platform. SignalPath's technology, much like Baseline, is designed to streamline and improve the efficiency of clinical trials.
Research Methodology
Our research methodology combines both qualitative and quantitative approaches to provide you with a thorough market analysis. We begin by gathering data from trusted industry reports and databases (secondary research), followed by primary research through surveys and interviews with key experts. We then apply advanced statistical tools to analyze the data, uncover trends, and assess market dynamics. Additionally, we use market segmentation and Porter's Five Forces analysis to evaluate competition. This approach ensures that the insights we provide are reliable, actionable, and tailored to support your decision-making process.
Make an Enquiry for purchasing this Report @ https://www.datamintelligence.com/enquiry/clinical-trial-management-system-market
Segment Covered in the Clinical Trial Management System Market:
By Type: On-Site CTMS, Enterprise-wide CTMS.
By Mode of Delivery: Web-based, On-premise, Cloud-based.
By Component: Software, Services.
By End User: Pharmaceuticals & Biotechnology Companies, CROs, Research Hospitals, Others.
This Report Covers:
✔ Go-to-market Strategy.
✔ Neutral perspective on the market performance.
✔Development trends, competitive landscape analysis, supply side analysis, demand side analysis, year-on-year growth, competitive benchmarking, vendor identification, Market Access, and other significant analysis, as well as development status.
✔Customized regional/country reports as per request and country level analysis.
✔ Potential & niche segments and regions exhibiting promising growth covered.
✔ Analysis of Market Size (historical and forecast), Total Addressable Market (TAM), Serviceable Available Market (SAM), Serviceable Obtainable Market (SOM), Market Growth, Technological Trends, Market Share, Market Dynamics, Competitive Landscape and Major Players (Innovators, Start-ups, Laggard, and Pioneer).
Regional Analysis for Clinical Trial Management System Market:
⇥ North America (U.S., Canada, Mexico)
⇥ Europe (U.K., Italy, Germany, Russia, France, Spain, The Netherlands and Rest of Europe)
⇥ Asia-Pacific (India, Japan, China, South Korea, Australia, Indonesia Rest of Asia Pacific)
⇥ South America (Colombia, Brazil, Argentina, Rest of South America)
⇥ Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of Middle East & Africa)
Benefits of the Report:
➡ A descriptive analysis of demand-supply gap, market size estimation, SWOT analysis, PESTEL Analysis and forecast in the global market.
➡ Top-down and bottom-up approach for regional analysis
➡ Porter's five forces model gives an in-depth analysis of buyers and suppliers, threats of new entrants & substitutes and competition amongst the key market players.
➡ By understanding the value chain analysis, the stakeholders can get a clear and detailed picture of this Market
Speak to Our Senior Analyst and Get Customization in the report as per your requirements: https://datamintelligence.com/customize/clinical-trial-management-system-market
Frequently asked questions:
➠ What is the global sales value, production value, consumption value, import and export of Clinical Trial Management System market?
➠ Who are the global key manufacturers of the Clinical Trial Management System Industry? How is their operating situation (capacity, production, sales, price, cost, gross, and revenue)?
➠ What are the Clinical Trial Management System market opportunities and threats faced by the vendors in the global Clinical Trial Management System Industry?
➠ Which application/end-user or product type may seek incremental growth prospects? What is the market share of each type and application?
➠ What focused approach and constraints are holding the Clinical Trial Management System market?
➠ What are the different sales, marketing, and distribution channels in the global industry?
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
About Us -
DataM Intelligence is a Market Research and Consulting firm that provides end-to-end business solutions to organizations from Research to Consulting. We, at DataM Intelligence, leverage our top trademark trends, insights and developments to emancipate swift and astute solutions to clients like you. We encompass a multitude of syndicate reports and customized reports with a robust methodology.
Our research database features countless statistics and in-depth analyses across a wide range of 6300+ reports in 40+ domains creating business solutions for more than 200+ companies across 50+ countries; catering to the key business research needs that influence the growth trajectory of our vast clientele.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Management System Market: Company Profiles, Segment Size, and Forecast Through 2024-2031 | Oracle, IQVIA Inc, BioClinica. here
News-ID: 3852788 • Views: …
More Releases from DataM Intelligence 4Market Research
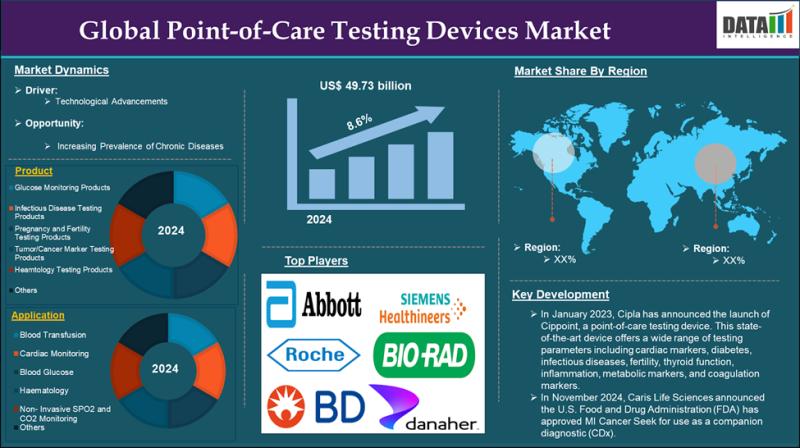
Point-of-Care Testing Devices Market is expected to reach US$ 101.51 billion by …
Market Size and Growth:
The Global Point-of-Care Testing Devices Market size reached US$ 49.73 billion in 2024 and is expected to reach US$ 101.51 billion by 2033, growing at a CAGR of 8.6% during the forecast period 2025-2033.
The Point-of-Care Testing Devices Market encompasses diagnostic tools and technologies that enable rapid, on-site medical testing near the patient, eliminating the need for centralized laboratories. These devices provide immediate results for various conditions, including…
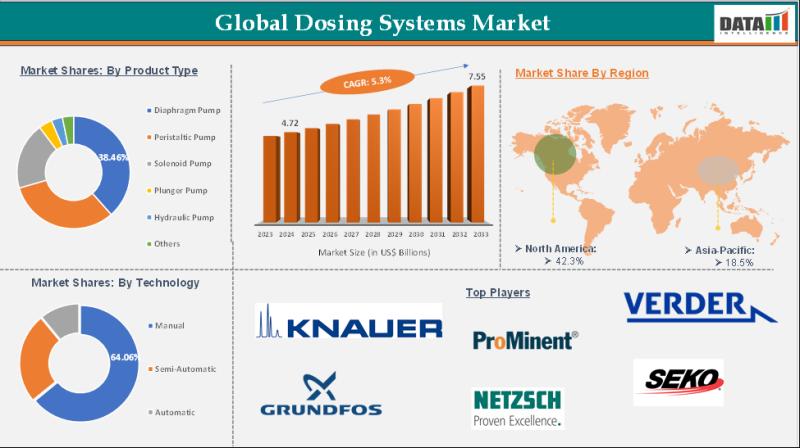
Dosing Systems Market is expected to reach US$ 7.55 Billion by 2033 | Major key …
Market Size and Growth:
The Dosing Systems Market size reached US$ 4.72 Billion in 2024 and is expected to reach US$ 7.55 Billion by 2033, growing at a CAGR of 5.3% during the forecast period 2025-2033.
The Dosing Systems Market encompasses the global industry involved in the development, manufacturing, and supply of precise fluid or chemical dosing equipment used across various sectors, including water treatment, chemicals, food & beverages, pharmaceuticals, and agriculture.…
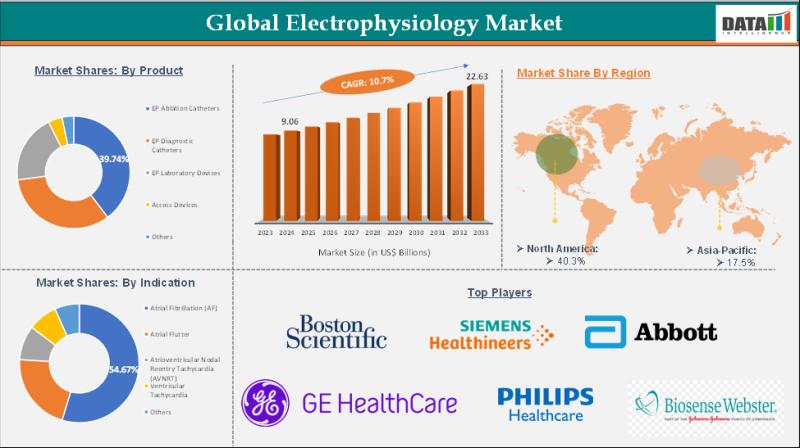
Electrophysiology Market is expected to reach US$ 22.63 Billion by 2033 | Major …
Market Size and Growth:
The Electrophysiology Market reached US$ 9.06 Billion in 2024 and is expected to reach US$ 22.63 Billion by 2033, growing at a CAGR of 10.7% during the forecast period 2025-2033.
The Electrophysiology Market encompasses the global industry focused on the diagnosis, monitoring, and treatment of heart rhythm disorders through advanced electrophysiology (EP) procedures, devices, and technologies. It includes EP catheters, mapping systems, ablation equipment, and implantable cardiac devices…
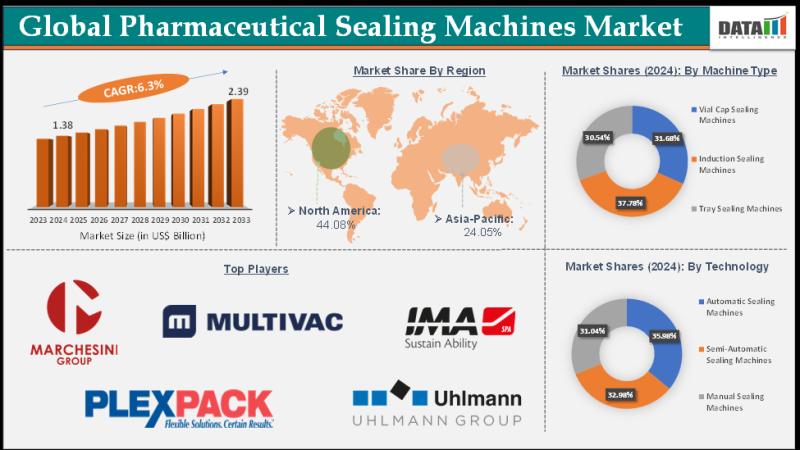
Pharmaceutical Sealing Machines Market is expected to reach US$ 2.39 Billion by …
Market Size and Growth:
The Pharmaceutical Sealing Machines Market size reached US$ 1.38 Billion in 2024 and is expected to reach US$ 2.39 Billion by 2033, growing at a CAGR of 6.3% during the forecast period 2025-2033.
The Pharmaceutical Sealing Machines Market encompasses the global industry involved in the manufacturing, distribution, and sale of machines designed to seal pharmaceutical products such as tablets, capsules, vials, bottles, and blister packs. These machines ensure…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…
