Press release
IVD Regulatory Affairs Outsourcing Market Scope: Growth, Share, Value, Size, and Analysis
"IVD Regulatory Affairs Outsourcing Market Size And Forecast by 2029Central to the analysis is the identification and evaluation of the Top 10 Companies in the IVD Regulatory Affairs Outsourcing Market. These organizations are recognized for their substantial market share and pivotal roles in driving industry growth. The report provides a detailed assessment of their business strategies, ranging from product development to market expansion efforts. It also highlights how these companies leverage technological advancements and market trends to maintain their leadership positions.
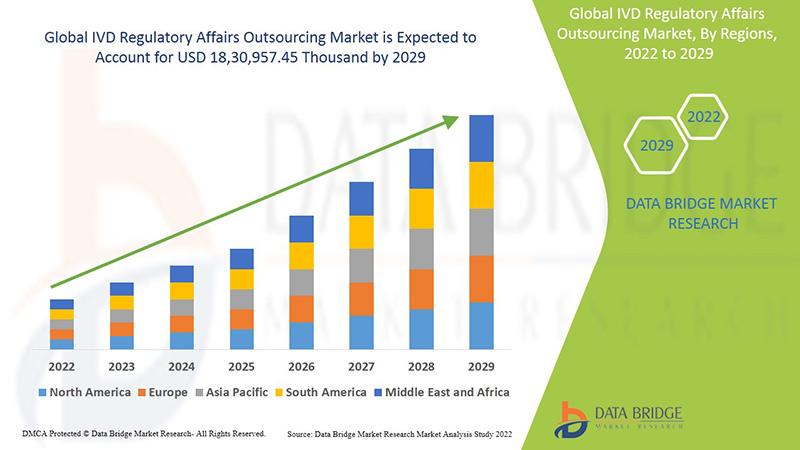
Global IVD regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with the CAGR of 13.3% in the forecast period of 2022 to 2029 and expected to reach USD 18, 30,957.45 thousand by 2029.
Explore Further Details about This Research IVD Regulatory Affairs Outsourcing Market Report https://www.databridgemarketresearch.com/reports/global-ivd-regulatory-affairs-outsourcing-market
Which are the top companies operating in the IVD Regulatory Affairs Outsourcing Market?
The Top 10 Companies in IVD Regulatory Affairs Outsourcing Market include well-established players. These companies are known for their market expertise, strong product portfolios, and significant market share. Their innovation, customer focus, and global operations have helped them maintain leadership positions in the market, offering high-quality solutions and services that meet the evolving needs of consumers.
**Segments**
- **Service Type:**
- Regulatory Writing and Publishing Services
- Regulatory Submissions
- Clinical Trial Application and Product Registrations
- Regulatory Consulting and Legal Representation
- Others
- **End-User:**
- IVD Manufacturers
- Biotechnology Companies
- CROs
- Academic Research Institutes
- Others
- **Region:**
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
**Market Players**
- **Key Players:**
- ICON plc
- PAREXEL International Corporation
- PPD, LLC
- WUXI Apptec
- Pharmaceutical Product Development, LLC
- Freyr Solutions
- TURNER Regulatory
- Dara AVISON Consulting
- Andaman Medical
- Kwizda Pharma GmbH
The IVD regulatory affairs outsourcing market is witnessing significant growth due to several factors. The complexity and rigidity of regulatory procedures have increased in the IVD industry, leading companies to seek outsourcing services. Regulatory writing and publishing services are in high demand as companies need assistance in compiling and submitting regulatory documents. Moreover, the growing number of clinical trials and the need for regulatory submissions have fueled the market for services like clinical trial application and product registrations. Regulatory consulting and legal representation play a crucial role in ensuring compliance with changing regulations, further driving the market growth.
In terms of end-users, IVD manufacturers are the key customers for regulatory affairs outsourcing services. These manufacturers rely on outsourcing partners to navigate the complex regulatory landscape and gain approvals for their products. Biotechnology companies and contract research organizations (CROs) also contribute significantly to the market demand for regulatory affairs outsourcing services. Academic research institutes are increasingly utilizing outsourcing services to streamline their regulatory processes and focus on core research activities.
Geographically, North America holds a substantial share in the IVD regulatory affairs outsourcing market. The presence of a large number of IVD companies in the region, coupled with stringent regulatory requirements, drives the demand for outsourcing services. Europe is another key market, with countries like Germany and the UK being major hubs for IVD manufacturers and regulatory affairs service providers. The market in Asia-Pacific is poised for significant growth due to the expanding healthcare sector and increasing investments in research and development.
Overall, the IVD regulatory affairs outsourcing market is dynamic and competitive, with key players like ICON plc, PAREXEL International Corporation, and WUXI Apptec leading the market. These companies offer a wide range of services catering to the diverse needs of IVD manufacturers and other end-users. With the increasing emphasis on regulatory compliance and quality standards in the IVD industry, the demand for outsourcing services is expected to continue growing.
The IVD regulatory affairs outsourcing market is experiencing a paradigm shift driven by factors such as technological advancements, evolving regulatory landscape, and increasing globalization of the healthcare industry. One notable trend is the rising demand for specialized regulatory services tailored to the IVD sector's unique requirements. Companies are seeking partners that not only understand the regulatory nuances of IVD products but also provide strategic guidance to navigate complex compliance challenges effectively.
Additionally, the convergence of digital health technologies with traditional IVD products is reshaping the regulatory environment and creating opportunities for service providers to offer innovative solutions. Regulatory writing and publishing services are evolving to encompass digital documentation and submission processes, reflecting the industry's move towards digitization and automation. This shift towards digital regulatory solutions is expected to streamline processes, enhance efficiency, and improve overall compliance outcomes for IVD manufacturers.
Another key aspect influencing the market dynamics is the changing regulatory landscape, marked by the adoption of new regulations and standards globally. As regulatory requirements become more stringent and complex, companies are turning to outsourcing partners with the expertise to interpret and implement these regulations effectively. Regulatory consulting and legal representation services are crucial in helping companies stay abreast of evolving regulatory changes and ensure compliance throughout the product lifecycle.
Moreover, the increasing focus on patient safety and product quality is driving the demand for robust regulatory strategies and comprehensive regulatory submissions. Service providers that offer end-to-end support in preparing, submitting, and maintaining regulatory documentation are well-positioned to capitalize on this trend. By providing a holistic approach to regulatory affairs outsourcing, companies can differentiate themselves in the market and attract clients looking for comprehensive regulatory solutions.
In conclusion, the IVD regulatory affairs outsourcing market is undergoing a transformative phase characterized by the demand for specialized services, digital innovation, and regulatory expertise. As the industry continues to evolve, service providers that can adapt to these changing dynamics and offer tailored solutions to meet the unique needs of IVD manufacturers will be poised for success. By leveraging technology, expertise, and industry knowledge, market players can drive efficiency, ensure compliance, and support the growth of the global IVD industry.**Segments**
Global IVD Regulatory Affairs Outsourcing Market, By Service:
- Regulatory Writing & Submissions
- Regulatory Registration & Clinical Trial Applications
- Regulatory Consulting
- Legal Representation
- Data Management Services
- Chemistry Manufacturing and Controls (CMC) Services
- Others
Indication:
- Oncology
- Neurology
- Cardiology
- Clinical Chemistry and Immunoassays
- Precision Medicine
- Infectious Diseases
- Diabetes
- Genetic Testing
- HIV/AIDS
- Haematology
- Drug Testing/Pharmacogenomics
- Blood Transfusion
- Point of Care
- Others
Deployment Mode:
- Cloud
- On-Premises
Organization Size:
- Small and Medium Enterprises (SMEs)
- Large Enterprises
Stage:
- Clinical
- Preclinical
- PMA (Post-Market Authorization)
Class:
- Class I
- Class II
- Class III
End User:
- Pharmaceutical Companies
- Medical Device Companies
- Biotechnology Companies
- Others
Country:
- U.S.
- Canada
- Mexico
- Germany
- France
- U.K.
- Italy
- Spain
- Netherlands
- Switzerland
- Russia
- Turkey
- Belgium
- Rest of Europe
- China
- South Korea
- Japan
- India
- Australia
- Singapore
- Malaysia
- Indonesia
- Thailand
- Philippines
- Rest of Asia-Pacific
- Saudi Arabia
- South Africa
- U.A.E.
- Egypt
- Israel
- Rest of Middle East and Africa
- Brazil
- Argentina
- Rest of South America
Industry Trends and Forecast to 2029.
**Market Players**
- Freyr Solutions
- PPD Inc. (A Subsidiary of Thermo Fisher Scientific Inc.)
- EMERGO
- ICON
- Parexel International Corporation
- CRITERIUM, INC.
- Groupe ProductLife S.A.
- Labcorp Drug Development
- WuXi AppTec
- Genpact
- Medpace
- Dor Pharmaceutical Services
- Qserve
The global IVD regulatory affairs outsourcing market is experiencing robust growth driven by various factors. Companies in the IVD sector are increasingly outsourcing regulatory affairs services due to the growing complexity and strictness of regulatory processes. The demand for services such as regulatory writing and publishing, clinical trial applications, and regulatory consulting is on the rise as companies seek assistance in navigating the regulatory landscape and ensuring compliance with evolving standards.
IVD manufacturers are the primary end-users of regulatory affairs outsourcing services, relying on external partners to guide them through regulatory requirements and approvals for their products. Biotechnology companies, CROs, and academic research institutes also significantly contribute to the market demand for outsourcing services as they seek support in regulatory compliance and submissions.
North America and Europe hold substantial shares in the market, with the presence of numerous IVD companies and stringent regulatory frameworks driving the need for outsourcing services. The Asia-Pacific market is witnessing significant growth due to the expanding healthcare sector and increased investments in R&D, presenting opportunities for market players to expand their operations in the region.
The market is highly competitive, with key players like Freyr Solutions, PPD Inc., ICON, and Parexel International Corporation leading the industry. These companies offer a wide array of services tailored to meet the specific needs of IVD manufacturers and other end-users. With technological advancements, evolving regulations, and globalization shaping the industry landscape, service providers offering specialized solutions, digital innovations, and comprehensive regulatory support are likely to thrive in the market. Competitive analysis plays a critical role in distinguishing the strengths of each market player and identifying opportunities for growth and differentiation in the dynamic IVD regulatory affairs outsourcing market.
Get More Details :
https://marketnews230.blogspot.com/2025/02/wood-coating-resins-market-graph-growth.html
https://marketnews230.blogspot.com/2025/02/hospital-sterilization-equipment-market.html
https://marketnews230.blogspot.com/2025/02/dairy-starter-culture-market-scope.html
https://marketnews230.blogspot.com/2025/02/automotive-connecting-rod-market-scope.html
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release IVD Regulatory Affairs Outsourcing Market Scope: Growth, Share, Value, Size, and Analysis here
News-ID: 3851573 • Views: …
More Releases from Data Bridge Market Research

Scented Candle Market Shows Strong Growth Driven by Wellness and Home Décor Tr …
The global scented candle market is on track for significant expansion, increasing from an estimated USD 3.60 billion in 2024 to USD 6.00 billion by 2032, registering a strong CAGR of 6.60%. Rising consumer interest in home ambiance, wellness, and premium lifestyle products continues to drive market demand.
Get More Detail: https://www.databridgemarketresearch.com/reports/global-scented-candle-market
Market Growth Drivers
The scented candle market has evolved beyond being just a decorative item. Key growth factors include:
Home Fragrance &…
Water Treatment System Market: Sustaining the Future of Clean Water
Introduction
Understanding Water Treatment Systems
Water treatment systems are designed to purify and disinfect water for various uses-drinking, industrial processes, irrigation, and wastewater reuse. These systems eliminate contaminants such as bacteria, viruses, heavy metals, chemicals, and particulates, making water safe and sustainable for consumption and use.
Importance in Global Sustainability
Clean water is essential to life and industrial progress. With growing water demand and pollution, water treatment systems are now critical infrastructure across the…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…