Press release
Exploring the Growth and Potential of the Clinical Trial Investigative Site Network Market
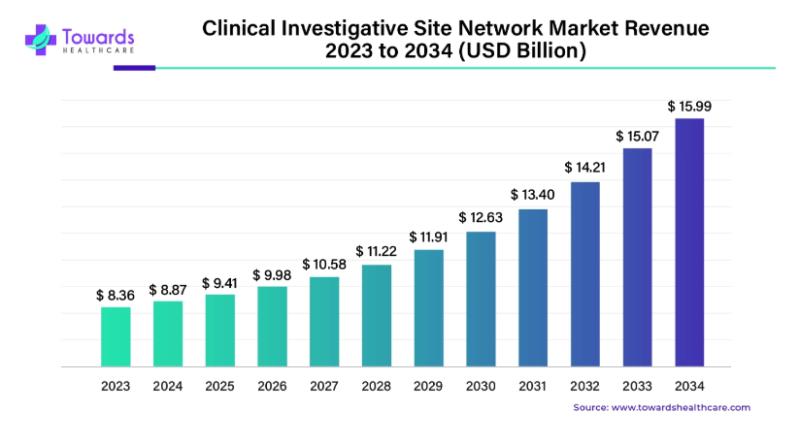
The clinical trial investigative site network market has become a pivotal element in the global clinical trial landscape, with its significant role in facilitating the research and development of novel therapies. In 2023, this market was valued at US$ 8.36 billion, with expectations to surge to US$ 15.99 billion by 2034. This robust growth, forecasted at a compound annual growth rate (CAGR) of 6.07%, is propelled by several key factors, including the increasing number of clinical trials, advancements in pharmaceutical and biotechnology research, and continuous technological innovations.Download Statistical Data: https://www.towardshealthcare.com/download-statistics/5236
Market Growth and Projections
The clinical trial investigative site network market has witnessed steady expansion and is expected to maintain its momentum over the next decade. The growing demand for faster drug development, coupled with the need for more efficient clinical trial management, has spurred the emergence of specialized networks dedicated to streamlining the trial process. As a result, the market is set to grow significantly, reaching nearly US$ 16 billion by 2034.
The factors driving this growth are multifaceted. Pharmaceutical and biotechnology companies are investing heavily in research to develop new treatments and therapies, leading to an increase in the number of clinical trials globally. Additionally, advancements in technology, including artificial intelligence (AI), data analytics, and cloud-based platforms, have enhanced the efficiency and accuracy of clinical trials, reducing timelines and improving outcomes.
Regional Insights: North America and Asia-Pacific Lead the Way
In 2023, North America commanded the largest market share, holding 52% of the global share. This dominance is largely due to the region's well-established infrastructure, highly regulated clinical trial environments, and a growing number of pharmaceutical companies conducting research and development activities. North America's robust healthcare systems and the presence of numerous Clinical Research Organizations (CROs) further contribute to its leadership in the market.
Meanwhile, Asia-Pacific is emerging as the fastest-growing region in the clinical trial investigative site network market, with an anticipated CAGR of 6.09% during the forecast period from 2024 to 2034. This rapid growth can be attributed to several factors, including the increasing number of clinical trials being outsourced to countries within the region, cost-effective clinical trial management, and an expanding pool of patients for clinical studies. Asia-Pacific's growth is also supported by improving healthcare infrastructure and regulatory reforms in key markets like China and India.
Therapeutic Areas Driving Market Growth
The oncology therapeutic area remains the dominant player in the market, accounting for 35% of the market share in 2023. This is not surprising, given the continued focus on cancer research and the ongoing need for new, more effective cancer therapies. Clinical trials in oncology are complex and require a large network of investigative sites to recruit diverse patient populations, making a centralized site network especially beneficial in streamlining these trials.
Pain management, however, is expected to see significant growth in the coming years. With increasing attention being paid to the global opioid crisis and the development of alternative pain management solutions, the pain management segment is poised to expand rapidly. This shift will likely result in an increase in clinical trials focused on chronic pain conditions, further propelling the demand for investigative site networks.
The Importance of Trial Phases: Phase III and I
In terms of clinical trial phases, Phase III trials accounted for 55% of the market share in 2023. These trials are typically the most extensive and resource-intensive, involving large patient populations to assess the effectiveness and safety of new drugs or treatments. The centralized structure of investigative site networks helps manage the complexities of Phase III trials, ensuring consistency across multiple locations and speeding up the overall process.
On the other hand, Phase I trials, which focus on the initial safety of a drug, are expected to see rapid expansion in the market. The growth of Phase I trials is driven by the increasing number of early-stage drugs entering clinical development, necessitating a robust investigative site network to manage these foundational trials.
End-Use Market: Sponsors and CROs
The sponsor segment dominated the clinical trial investigative site network market in 2023, with a 65% market share. Sponsors, including pharmaceutical and biotechnology companies, often rely on site networks to streamline their clinical trials. These organizations provide the necessary infrastructure to conduct trials efficiently, ensuring compliance with regulatory standards and delivering high-quality data.
However, the Contract Research Organization (CRO) segment is projected to gain significant market share during the forecast period from 2024 to 2034. As pharmaceutical and biotech companies increasingly outsource their clinical trials to CROs, the demand for clinical trial site networks will continue to grow. CROs play a crucial role in coordinating trials across multiple sites, making them a natural partner for investigative site networks.
The Role of Clinical Trial Investigative Site Networks in Streamlining Research
At the heart of the clinical trial investigative site network market lies the role of organizations that specialize in facilitating the successful completion of clinical trials. These organizations provide an array of services, including site identification, financial management, regulatory compliance, data management, and safety monitoring. By centralizing administrative functions, investigative site networks enable pharmaceutical and biotech companies to focus on research while reducing the complexities associated with managing multiple sites.
Centralization of these services offers several advantages. For instance, it reduces the number of contacts between sponsors and clinical sites, streamlining communication and enhancing the speed of decision-making. Furthermore, by integrating site feasibility studies, financial management, and contracts into a unified system, site networks significantly cut down on study startup times, allowing trials to progress more efficiently.
This integrated approach also improves patient centricity, ensuring that patients receive timely and high-quality care throughout the trial process. By resolving long-standing process and workflow challenges, clinical trial investigative site networks contribute to the safe and effective delivery of therapies to the market, benefiting both patients and the broader healthcare community.
Harnessing the Power of AI to Revolutionize the Clinical Trial Investigative Site Network Market
The clinical trial process has long been recognized as a complex and resource-intensive journey. From the intricate coordination of multiple sites to the recruitment and monitoring of patients, clinical trials are often time-consuming, costly, and require extensive data management. However, the advent of artificial intelligence (AI) is reshaping this landscape, offering transformative solutions that streamline operations, enhance efficiency, and improve outcomes. AI is now a key player in clinical trials, with a particular impact on the clinical trial investigative site network market.
As the healthcare industry faces increasing pressure to reduce costs and accelerate the development of new therapies, AI is poised to be a game-changer in clinical trials. By leveraging the power of AI, organizations can automate and optimize various stages of the clinical trial process, from site selection to patient recruitment and data analysis, ensuring faster, more accurate results. Here's how AI is improving the clinical trial investigative site network market.
Streamlining Site and Investigator Identification
One of the most labor-intensive aspects of clinical trials is identifying the right sites and investigators. Traditionally, this process involves manually sifting through numerous records, evaluating the capabilities of clinical sites, and assessing the experience of investigators. AI, however, can automate this process by analyzing large datasets to identify the most suitable sites and investigators based on the specific requirements of a research study. By examining historical data, trial results, and site performance metrics, AI can predict the likelihood of success for a given site or investigator, enabling more informed decision-making.
This data-driven approach significantly reduces the time and effort involved in site and investigator selection, ensuring that the clinical trial is set up for success from the start. AI can also predict potential challenges that may arise at different sites, enabling proactive planning and mitigating risks before they become issues.
Enhancing Patient Recruitment and Retention
Another critical challenge in clinical trials is recruiting the right patient population. Traditionally, patient recruitment has been a slow and costly process, often involving manual screening and outreach. AI can revolutionize this process by analyzing electronic health records (EHRs), genetic data, and other patient databases to identify individuals who meet the specific criteria for a study. By using AI-powered algorithms, researchers can target the most relevant patients, improving recruitment efficiency and ensuring that trials are conducted with the appropriate participants.
Beyond recruitment, AI can also help optimize patient retention. By analyzing patient data and identifying patterns, AI can predict potential dropouts and suggest interventions to improve patient engagement and retention throughout the trial. This not only saves time but also ensures that clinical trials are completed on schedule, with minimal disruptions.
Decentralized Clinical Trials: AI's Role in Remote Monitoring
The rise of decentralized clinical trials (DCTs) has further emphasized the importance of AI in clinical trials. DCTs enable patients to participate in trials from the comfort of their homes, reducing the need for travel and in-person visits to clinical sites. AI plays a crucial role in ensuring that these trials run smoothly by remotely monitoring patient health and collecting data through wearables and other connected devices. This real-time data is processed by AI systems, allowing for continuous assessment of patient conditions and enabling timely interventions when necessary.
AI can also optimize the dosage of treatments based on individual patient responses, ensuring that each participant receives the most effective and personalized care. By analyzing patient data in real-time, AI helps researchers make more informed decisions about treatment adjustments, potentially improving the overall success rate of the trial.
Data Integration and Analysis for Informed Decision-Making
The sheer volume of data generated during clinical trials can be overwhelming, making it difficult to extract meaningful insights. AI can efficiently process massive datasets, integrating data from multiple sources, such as patient records, trial results, lab tests, and medical imaging. Once integrated, AI systems can interrogate and analyze this data to identify patterns, correlations, and trends that might otherwise go unnoticed.
By leveraging AI-driven data analysis, clinical trial stakeholders can make more informed decisions at every stage of the process, from study design to monitoring and reporting. AI can also aid in detecting anomalies or adverse reactions in real time, enabling swift interventions that improve patient safety and trial outcomes.
Optimizing Decision-Making Across the Trial Process
AI's ability to analyze large volumes of data in real time also enhances decision-making capabilities throughout the clinical trial process. For example, AI-powered platforms can monitor the progress of trials, providing insights into whether they are on track and if any adjustments need to be made. This continuous feedback loop enables clinical trial sponsors, contract research organizations (CROs), and investigative site networks to make data-driven decisions quickly, optimizing the trial's success.
Additionally, AI can assist in optimizing treatment protocols, identifying the most effective treatment plans based on individual patient data. By continuously analyzing data from ongoing trials, AI can help researchers refine protocols and improve the chances of achieving positive clinical outcomes.
AI-Powered Future of Clinical Trial Investigative Site Networks
As clinical trials become increasingly complex, the need for advanced technologies like AI will continue to grow. The integration of AI into the clinical trial investigative site network market is helping to streamline the entire clinical trial process, from planning and recruitment to execution and analysis. By automating labor-intensive tasks and providing deeper insights into trial data, AI is making clinical trials more efficient, cost-effective, and ultimately more successful.
Furthermore, the continued evolution of AI technologies promises even greater advancements in the years to come. As AI algorithms become more sophisticated and data sources become more abundant, the potential for AI to improve clinical trial operations will expand, opening up new opportunities for faster drug development and more personalized patient care.
Source: https://www.towardshealthcare.com/insights/clinical-trial-investigative-site-network-market-sizing
Baner
Buy Premium Global Insight: https://www.towardshealthcare.com/price/5236
Review the Full TOC for the Clinical Trial Investigative Site Network Market Report: https://www.towardshealthcare.com/table-of-content/clinical-trial-investigative-site-network-market-sizing
Get the latest insights on packaging industry segmentation with our Annual Membership - https://www.towardspackaging.com/get-an-annual-membership
About Us
Towards Packaging is a leading global consulting firm specializing in providing comprehensive and strategic research solutions. With a highly skilled and experienced consultant team, we offer a wide range of services designed to empower businesses with valuable insights and actionable recommendations. We stay abreast of the latest industry trends and emerging markets to provide our clients with an unrivalled understanding of their respective sectors. We adhere to rigorous research methodologies, combining primary and secondary research to ensure accuracy and reliability. Our data-driven approach and advanced analytics enable us to unearth actionable insights and make informed recommendations. We are committed to delivering excellence in all our endeavours. Our dedication to quality and continuous improvement has earned us the trust and loyalty of clients worldwide.
Browse our Brand-New Journal:
Towards Healthcare: https://www.towardshealthcare.com
Towards Automotive: https://www.towardsautomotive.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-packaging/
Get Our Freshly Printed Chronicle: https://www.packagingwebwire.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Exploring the Growth and Potential of the Clinical Trial Investigative Site Network Market here
News-ID: 3810231 • Views: …
More Releases from Towards Healthcare

Rapid Advancements in Cardiac Biomarkers Shaping the Future of Healthcare
The global cardiac biomarkers market is experiencing substantial growth, driven by a surge in the number of cardiovascular diseases (CVDs) and a rising demand for early diagnostics and preventive care. Valued at USD 21.27 billion in 2024, the market is projected to grow to USD 24.39 billion in 2025 and reach an impressive USD 83.54 billion by 2034, expanding at a compound annual growth rate (CAGR) of 14.66% during this…

Rapid Advancements in Cardiac Biomarkers Shaping the Future of Healthcare
The global cardiac biomarkers market is experiencing substantial growth, driven by a surge in the number of cardiovascular diseases (CVDs) and a rising demand for early diagnostics and preventive care. Valued at USD 21.27 billion in 2024, the market is projected to grow to USD 24.39 billion in 2025 and reach an impressive USD 83.54 billion by 2034, expanding at a compound annual growth rate (CAGR) of 14.66% during this…

Exploring the Impact of Robotics on the Dental Industry
The field of robotic dentistry is rapidly evolving, with technological advancements and the increasing prevalence of dental diseases driving substantial growth. Valued at an estimated US$ 535 million in 2023, the robotic dentistry market is poised to reach US$ 2.58 billion by 2034, growing at an impressive compound annual growth rate (CAGR) of 15.4% from 2024 to 2034. This surge is driven by a combination of factors, including innovation in…

Revolutionizing Industries with Key Developments in the Microbial Fermentation T …
The microbial fermentation technology market is rapidly expanding, reflecting a broader shift towards sustainable and bio-based manufacturing processes. Valued at approximately USD 34.11 billion in 2023, the market is set to experience significant growth, with projections placing its value at USD 60.17 billion by 2033. This growth is anticipated at a compound annual growth rate (CAGR) of 5.84% from 2024 to 2033. The rise in demand for biologics, coupled with…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…