Press release
Medical Device Package Validation Market Detailed Analysis with Accurate Forecast to 2031
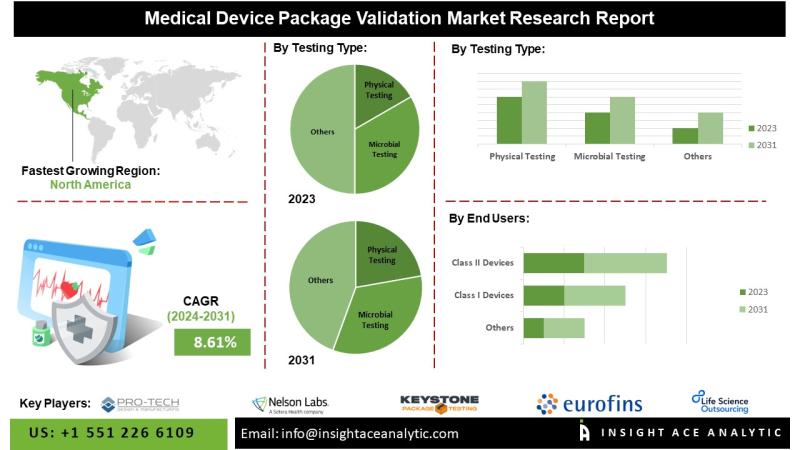
Medical Device Package Validation Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce AnalyticInsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Medical Device Package Validation Market- (By Testing Type (Physical Testing, Microbial Testing, Chemical Testing, and Visual Testing), By Device Class (Class I Devices, Class II Devices, and Class III Devices)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Medical Device Package Validation Market is to grow with a CAGR of 8.61% during the forecast period of 2024-2031.
Get Free Access to Demo Report, Excel Pivot and ToC: www.insightaceanalytic.com/request-sample/2701
Packaging validation is an all-encompassing procedure that involves identifying and controlling the materials and manufacturing factors that impact the capability of a packaged item to fulfil its acceptance qualifications. Several advantages are produced as a consequence of the validation process. It is possible to accomplish process control and the assurance that the device package criteria will be met by determining the optimal windows for each key variable based on the information obtained. To guarantee the safety, effectiveness, and integrity of medical devices throughout their entire lifespan, severe regulatory requirements are the primary force behind the market for medical device package validation. Regulatory authorities such as the FDA and the CE mark require comprehensive validation procedures to reduce the hazards connected with packaging failures and to guarantee that goods continue to be sterile and safe for patient use. This regulatory scrutiny pushes medical device makers to invest in secure packaging validation solutions that are in accordance with international standards, which ultimately results in the expansion of the industry. An additional factor that significantly contributes to the formation of the market landscape is the development of new technologies and materials for packaging. There is a growing interest in innovations such as active and intelligent packaging solutions, which can monitor and preserve the integrity of the product. At the same time, it is being stored and transported. In addition to improving product safety and extending the shelf life of products, these technologies also improve packaging performance.
Expert Knowledge, Just a Click Away:https://calendly.com/insightaceanalytic/30min?month=2024-02
List of Prominent Players in the Medical Device Package Validation Market:
• Wetspak
• Life Science Outsourcing, Inc.
• Pro-Tech Design & Manufacturing
• WuXi AppTec Medical Device Testing
• Nelson Labs
• Keystone Package Testing
• Eurofins Scientific
• UL Solutions
• SteriPack Contract Manufacturing
• DDL, Inc.
• Other Prominent Players
Market Dynamics:
Drivers:
Growing consumer concern regarding spreading contagious diseases drives the medical device package validation market. The validation of medical device packaging is a significant factor in improving patient safety and fostering customer trust. Failures in packaging that might put the safety of patients at risk, including leaks, breaches, and poor labelling, can be avoided with the participation of validation. Additionally, it gives patients and healthcare professionals the certainty that the medical equipment they use is safe, dependable, and sufficiently high quality. Additionally, validation is a proactive strategy to detect and resolve possible difficulties, limit risks, and constantly improve package designs and processes. Validation is helpful for all of these purposes.
Challenges:
The prime challenge is inadequate comprehension of packing material characteristics and challenges related to documentation and record-keeping, which are predicted to reduce the growth of the medical device package validation market. When it comes to showing compliance and providing a reference for an audit or inspection, thorough documentation and record-keeping are necessary. Standard operating procedures (SOPs), protocols, test findings, and validation reports might need to be simplified to maintain detailed documentation. This can be a challenge. It might also be difficult to choose suitable materials for specific medical devices due to the intricacy of the materials used for packaging and the features that they possess. Manufacturers need to have a comprehensive grasp of the characteristics of the materials, as well as how those qualities interact with the device and the approaches to sterilization.
Regional Trends:
North America is anticipated to be the most significant region in the global medical device packaging validation market. Its comprehensive healthcare facilities, stringent regulatory frameworks, such as FDA requirements, and the preponderance of prominent medical device manufacturers are the reasons for the market's dominance. Comprehensive package validation services are in high demand throughout the region due to these factors, which guarantee adherence to stringent quality and safety standards. Moreover, The Asia Pacific region is expected to become the fastest-growing region worldwide for validating medical device packaging. The expansion of healthcare expenditures drives the fast development of the regional market, the growing acceptance of medical devices, and the increasing awareness of patient safety and regulatory compliance.
Unlock Your GTM Strategy: www.insightaceanalytic.com/customisation/2701
Recent Developments:
• In February 2024, Berry Global Inc. has declared its intention to separate and combine its Health, Hygiene, and Specialties Global Nonwovens and Films (HHNF) division with Glatfelter Corporation.
• In January 2024, Amcor Plc has declared the augmentation of its thermoforming manufacturing capacity in order to meet the growing demand from clients in the medical, pharmaceutical, and consumer health industries. on the continent of North America.
• In August 2023, DuPont de Nemours, Inc. has successfully acquired Spectrum Plastics Group from AEA Investors. Spectrum Plastics Group is a renowned industry leader in the innovative manufacture of specialized medical devices and components.
Segmentation of Medical Device Package Validation Market
Medical Device Package Validation Market- By Testing Type
• Physical Testing
• Microbial Testing
• Chemical Testing
• Visual Testing
Medical Device Package Validation Market- By Device Class
• Class I Devices
• Class II Devices
• Class III Devices
Medical Device Package Validation Market- By Region
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Empower Your Decision-Making with 180 Pages Full Report @ www.insightaceanalytic.com/buy-report/2701
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Medical Device Package Validation Market Detailed Analysis with Accurate Forecast to 2031 here
News-ID: 3809324 • Views: …
More Releases from InsightAce Analytic Pvt. Ltd.
High-Temperature Fuel Cells Market Benefits from Technological Advancements and …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global High-Temperature Fuel Cells Market- (By Type (Solid Oxide Fuel Cell, Molten Carbonate Fuel Cell, and Others); By Application (Transportation, Distributed Generation and Others)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global High-Temperature Fuel Cells Market is expected to grow with a CAGR of…

Organic Feminine Care Market to Benefit from Increasing Government Initiatives a …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Organic Feminine Care Market Size, Share & Trends Analysis Report By Product Type (Sanitary Pads, Tampons, Menstrual Cups, Liners and Shields, Others), by Nature (Disposable, Reusable), by Age Group (Upto 18 Years, 19-30 Years, 31-40 Years, 41 Years and Above), by Distribution Channel (Supermarkets and hypermarkets, Pharmacy, Online Stores, Others)- Market Outlook And Industry Analysis…

Intravenous Immunoglobulin Market Key Players Analysis - Biotest AG, Octapharma …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Intravenous Immunoglobulin Market Size, Share & Trends Analysis Report By Application (Hypogammaglobulinemia, CIDP, Congenital AIDS), By Distribution Channel (hospital pharmacies, speciality pharmacies), Region, Market Outlook And Industry Analysis 2031"
The global Intravenous Immunoglobulin market is estimated to reach over USD 21.22 billion by 2031, exhibiting a CAGR of 6.60% during the forecast period.
Get Free Access…

Exascale Computing Market Key Players Analysis- Hewlett Packard Enterprise Compa …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Exascale Computing Market - (By Component (Hardware, Software, Services), By Deployment (On-premises, Cloud-based), By Customer Type (Government & Defense, Healthcare & Biosciences, Financial Services, Research & Academia, Manufacturing & Energy, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Exascale Computing Market is valued at…
More Releases for Device
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Surge In Wireless Device Usage Boosts Wireless Audio Device Market Driving Marke …
Stay ahead with our updated market reports featuring the latest on tariffs, trade flows, and supply chain transformations.
How Large Will the Wireless Audio Device Market Size By 2025?
In recent years, there has been remarkable growth in the wireless audio device market size. The market, which is projected to expand from $41.85 billion in 2024 to $52.37 billion in 2025, boasts a compound annual growth rate (CAGR) of 25.1%. Factors contributing…
Anti-snoring Device Market - Quiet Nights, Restful Sleep: Anti-snoring Device In …
Newark, New Castle, USA: The "Anti-snoring Device Market" provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors.
Anti-snoring Device Market: https://www.growthplusreports.com/report/antisnoring-device-market/8931
This latest report researches the industry structure, sales, revenue,…
Global Watch Clock Measuring Device Market | Watch Clock Measuring Device Indust …
Watch, clock and measuring device market comprises of the sales of watch, clock, measuring device & related services to measure the time and physical quantity. Watch is portable timepiece, which is worn by people around the wrist, attached by a strap. Clock is a device used to measure and indicate time, using the pointers moving over a dial. Measuring device is an instrument used for measuring the various parameters in…
Peripheral Vascular Device Market Size, Peripheral Vascular Device Market Share, …
Global Peripheral Vascular Device Market Size is observed to gain traction owing to the factors such as increasing research and development for developing several new product, and rising funding by the private organizations.
Request for Sample of This Research Report @ https://bit.ly/2xjOKpC
Top Key Player:-
Abbott Laboratories
Braun Melsungen AG
Boston Scientific Corporation
R. Brad, Inc.
Cardinal Health, Inc.
Medtronic plc.
Cook Medical, Inc.
Teruma Corporation
Jude Medical, Inc.
The Spectranetics Corporation
Volcano Corporation
Peripheral vascular disorder (PVD) is a blood circulation disorder…
Medical Device Technologies Market - The Evolution of Medical Device Technologie …
The global medical device technologies market is anticipated to be boosted by various well-known players in the market. Some of these players that are dealing with the manufacturing of in vitro diagnostic devices hold a significant share in the global market. Whereas, the small market players are emerging from several developing nations, looking to set their foot in the market. Such measures are foreseen to change the market scenario in…