Press release
Cell Harvesting System Market: Regulatory Trends and Compliance in the Biotechnology Sector
𝐈𝐧𝐭𝐫𝐨𝐝𝐮𝐜𝐭𝐢𝐨𝐧Cell harvesting systems play a pivotal role in the biotechnology and healthcare industries. These systems are essential in the collection of cells for various applications, such as stem cell research, gene therapy, and cancer treatments. With the growth of cell-based therapies and regenerative medicine, the demand for advanced cell harvesting technologies has surged. However, this rapid growth also brings the need for stringent regulatory frameworks and compliance measures to ensure safety, efficacy, and ethical considerations in biotechnological innovations.
In this article, we will explore the regulatory trends and compliance requirements in the cell harvesting system market, how they shape the development and adoption of these technologies, and the role of key stakeholders in navigating the regulatory landscape.
The global cell harvesting system market is estimated to value at US$14.3 Bn by the end of 2031 from US$6.2 Bn recorded in 2024. The market is projected to secure a CAGR of 12.2% in the forthcoming years from 2024 to 2031. North America currently dominates the market, followed by Europe and Asia-Pacific, owing to robust healthcare infrastructure and rising investments in biotechnology. The market is expected to expand as more healthcare providers adopt automated cell harvesting technologies to improve efficiency and precision.
𝐆𝐞𝐭 𝐚 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐁𝐫𝐨𝐜𝐡𝐮𝐫𝐞 𝐨𝐟 𝐭𝐡𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 (𝐔𝐬𝐞 𝐂𝐨𝐫𝐩𝐨𝐫𝐚𝐭𝐞 𝐄𝐦𝐚𝐢𝐥 𝐈𝐃 𝐟𝐨𝐫 𝐚 𝐐𝐮𝐢𝐜𝐤 𝐑𝐞𝐬𝐩𝐨𝐧𝐬𝐞): https://www.persistencemarketresearch.com/samples/34752
𝐓𝐡𝐞 𝐑𝐨𝐥𝐞 𝐨𝐟 𝐂𝐞𝐥𝐥 𝐇𝐚𝐫𝐯𝐞𝐬𝐭𝐢𝐧𝐠 𝐒𝐲𝐬𝐭𝐞𝐦𝐬 𝐢𝐧 𝐁𝐢𝐨𝐭𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐲
Cell harvesting systems are designed to efficiently and safely collect cells from various sources, including blood, tissues, and bone marrow. These systems are critical in fields such as:
• Stem Cell Therapy: Cells are harvested for regenerative medicine and tissue engineering.
• Gene Therapy: Harvested cells are used for genetic modifications and treatments.
• Cancer Treatment: Autologous or allogeneic cells are harvested to develop personalized cancer treatments.
• Vaccine Production: Cells are harvested to produce viral vectors for vaccines.
The effectiveness of these treatments depends not only on the harvesting system's technology but also on adherence to strict regulatory requirements that ensure the integrity and safety of the cells used in clinical and therapeutic settings.
Regulatory Trends in the Cell Harvesting System Market
As the biotechnology sector expands, the regulatory environment becomes increasingly complex. Various regulatory bodies around the world oversee the approval and monitoring of cell harvesting systems. In North America, the U.S. Food and Drug Administration (FDA) and Health Canada are the primary authorities.
𝐁𝐞𝐥𝐨𝐰 𝐚𝐫𝐞 𝐬𝐨𝐦𝐞 𝐤𝐞𝐲 𝐫𝐞𝐠𝐮𝐥𝐚𝐭𝐨𝐫𝐲 𝐭𝐫𝐞𝐧𝐝𝐬 𝐬𝐡𝐚𝐩𝐢𝐧𝐠 𝐭𝐡𝐞 𝐜𝐞𝐥𝐥 𝐡𝐚𝐫𝐯𝐞𝐬𝐭𝐢𝐧𝐠 𝐬𝐲𝐬𝐭𝐞𝐦 𝐦𝐚𝐫𝐤𝐞𝐭:
1. Stricter Compliance and Safety Protocols
With the increasing use of cell-based therapies, there is a heightened focus on ensuring that cell harvesting systems meet rigorous safety standards. The FDA, for instance, enforces regulations around Good Manufacturing Practices (GMP), which are designed to ensure the quality and safety of medical devices, including cell harvesting systems. GMP compliance involves stringent controls over the manufacturing, processing, and packaging of devices to prevent contamination, ensure traceability, and minimize risk.
2. Accelerating Approvals for Innovative Therapies
Regulatory bodies are adopting faster approval processes to accelerate the introduction of cutting-edge technologies in cell therapy. For example, the FDA's Regenerative Medicine Advanced Therapy (RMAT) designation fast-tracks cell therapies that show promise for treating serious conditions. This has significant implications for cell harvesting systems, as companies develop devices capable of efficiently collecting and processing cells for these rapidly advancing treatments.
3. Integration of Artificial Intelligence and Automation
Advanced automation and AI-driven solutions are being integrated into cell harvesting systems, enhancing their precision and efficiency. Regulatory authorities are working to ensure that these technologies meet new standards related to data integrity, cybersecurity, and machine learning algorithms. The FDA's emphasis on Software as a Medical Device (SaMD) has led to the establishment of new guidelines that specifically address the risks and safety concerns associated with AI-powered systems.
4. Ethical Considerations in Stem Cell Research
Ethical issues surrounding the use of stem cells, especially from embryos, have influenced regulatory trends in the biotechnology sector. Regulatory bodies such as the FDA and European Medicines Agency (EMA) have developed guidelines to address these concerns while facilitating research and innovation in regenerative medicine. In particular, there is a concerted effort to ensure that the use of human cells in clinical trials and treatments adheres to ethical standards regarding consent, privacy, and the sustainability of the cell sources.
5. International Regulatory Harmonization
The global nature of biotechnology has led to the need for regulatory harmonization across different regions. Organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the World Health Organization (WHO) are working to standardize regulations for cell harvesting and therapy systems worldwide. These efforts aim to facilitate the global movement of cell therapies and technologies while maintaining high standards of safety and efficacy.
Compliance Requirements for Cell Harvesting Systems
Compliance with regulatory guidelines is not optional but a critical component of the development, manufacturing, and marketing of cell harvesting systems.
𝐁𝐞𝐥𝐨𝐰 𝐚𝐫𝐞 𝐤𝐞𝐲 𝐜𝐨𝐦𝐩𝐥𝐢𝐚𝐧𝐜𝐞 𝐫𝐞𝐪𝐮𝐢𝐫𝐞𝐦𝐞𝐧𝐭𝐬 𝐭𝐡𝐚𝐭 𝐜𝐨𝐦𝐩𝐚𝐧𝐢𝐞𝐬 𝐦𝐮𝐬𝐭 𝐚𝐝𝐡𝐞𝐫𝐞 𝐭𝐨:
1. FDA Regulations (U.S.)
The FDA plays a central role in regulating medical devices, including cell harvesting systems. The following are the primary regulatory frameworks that impact the market:
• 21 CFR Part 820: This regulation outlines the FDA's Quality System Regulations (QSR) for medical device manufacturers, ensuring that cell harvesting systems meet the necessary quality standards throughout their lifecycle.
• Premarket Notification (510(k)): Companies wishing to introduce new cell harvesting systems to the market must submit a 510(k) notification to the FDA, demonstrating that their device is substantially equivalent to an already approved device.
• Investigational Device Exemption (IDE): If a company intends to conduct clinical trials using a cell harvesting system, they must obtain an IDE from the FDA to ensure the system meets clinical standards.
2. Health Canada Regulations (Canada)
Health Canada's Medical Device Regulations (MDR) govern the approval of cell harvesting systems in Canada. The MDR mandates that all medical devices, including those used in cell therapy, comply with the Canadian Medical Devices Conformity Assessment System (CMDCAS) to ensure they meet the necessary standards of safety, effectiveness, and quality.
• Class II, III, and IV Devices: Depending on the risk associated with the device, cell harvesting systems must be classified and undergo appropriate assessments.
• Licensing and Inspections: Manufacturers must hold a Medical Device License (MDL) and undergo regular inspections to ensure continued compliance with regulatory standards.
3. European Union Medical Device Regulations (MDR)
In the European Union, the new Medical Device Regulation (MDR 2017/745), which came into effect in May 2021, has placed more emphasis on the traceability and safety of medical devices. This regulation applies to all medical devices, including cell harvesting systems, ensuring their safety for patients and clinical settings. Manufacturers must ensure that their systems meet the new clinical evaluation requirements and undergo CE marking to demonstrate compliance with EU regulations.
The Impact of Consumer Awareness on Regulatory Trends
Consumer awareness plays a pivotal role in shaping regulatory trends in the biotechnology sector. As patients and healthcare providers become more knowledgeable about the benefits and risks of cell-based therapies, they are demanding higher standards of transparency, accountability, and safety.
• Educated Consumers: With increasing awareness of the potential of stem cell and gene therapies, consumers are becoming more vocal in demanding stringent safety standards for cell harvesting systems. This shift has encouraged regulators to adopt more robust and comprehensive guidelines that protect patient safety while facilitating innovation.
• Ethical Concerns: The rise of consumer advocacy groups focused on ethical issues in biotechnology, including stem cell research and gene editing, has forced regulators to address these concerns in their policies. Ethical transparency, informed consent, and sustainable sourcing of cells have become key regulatory concerns, influenced by consumer pressures.
• Globalization and Information Access: The advent of digital platforms has allowed consumers to access information about the latest biotechnology developments. This has led to greater public scrutiny and the need for clear regulatory standards that are easily understood and accessible.
𝐂𝐨𝐧𝐜𝐥𝐮𝐬𝐢𝐨𝐧
The cell harvesting system market is poised for substantial growth as the biotechnology sector continues to evolve and advance. Regulatory trends and compliance measures are integral in ensuring that these systems are safe, effective, and ethical for use in clinical settings. As consumer awareness grows, there is an increased demand for higher safety standards, transparency, and accountability in biotechnology. Regulatory bodies are responding by refining existing frameworks and adopting new guidelines to meet the challenges of an expanding market. For companies in the biotechnology sector, understanding and complying with these regulations is essential to gaining market approval and fostering consumer trust.
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐭𝐡𝐞 𝐋𝐚𝐭𝐞𝐬𝐭 𝐓𝐫𝐞𝐧𝐝𝐢𝐧𝐠 "𝐄𝐱𝐜𝐥𝐮𝐬𝐢𝐯𝐞 𝐀𝐫𝐭𝐢𝐜𝐥𝐞":
• https://www.linkedin.com/pulse/how-industrial-racking-system-market-evolving-rise-aishvarya-doiphode-gi4uf/
• https://www.linkedin.com/pulse/animal-drug-compounding-addressing-needs-pet-owners-doiphode-tdiwf/
• https://www.linkedin.com/pulse/eeg-devices-market-how-brainwave-monitoring-transforming-l8fif/
• https://www.linkedin.com/pulse/europes-personalized-medicine-biomarkers-market-enphf/
• https://www.linkedin.com/pulse/ship-to-shore-sts-container-cranes-market-innovations-doiphode-vrwif/
• https://www.linkedin.com/pulse/north-america-orthokeratology-market-trends-growth-1tfnf/
• https://www.linkedin.com/pulse/mining-remanufacturing-component-market-sustainable-heavy-doiphode-45m8f/
• https://www.linkedin.com/pulse/asia-pacific-dental-crowns-bridges-market-increasing-cxoqf/
𝐀𝐛𝐨𝐮𝐭 𝐏𝐞𝐫𝐬𝐢𝐬𝐭𝐞𝐧𝐜𝐞 𝐌𝐚𝐫𝐤𝐞𝐭 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡:
At Persistence Market Research, we specialize in creating research studies that serve as strategic tools for driving business growth. Established as a proprietary firm in 2012, we have evolved into a registered company in England and Wales in 2023 under the name Persistence Research & Consultancy Services Ltd. With a solid foundation, we have completed over 3600 custom and syndicate market research projects, and delivered more than 2700 projects for other leading market research companies' clients.
Our approach combines traditional market research methods with modern tools to offer comprehensive research solutions. With a decade of experience, we pride ourselves on deriving actionable insights from data to help businesses stay ahead of the competition. Our client base spans multinational corporations, leading consulting firms, investment funds, and government departments. A significant portion of our sales comes from repeat clients, a testament to the value and trust we've built over the years.
𝐂𝐨𝐧𝐭𝐚𝐜𝐭 𝐔𝐬:
Persistence Market Research
G04 Golden Mile House, Clayponds Lane
Brentford, London, TW8 0GU UK
USA Phone: +1 646-878-6329
UK Phone: +44 203-837-5656
Email: sales@persistencemarketresearch.com
Web: https://www.persistencemarketresearch.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Cell Harvesting System Market: Regulatory Trends and Compliance in the Biotechnology Sector here
News-ID: 3807398 • Views: …
More Releases from Persistence Market Research
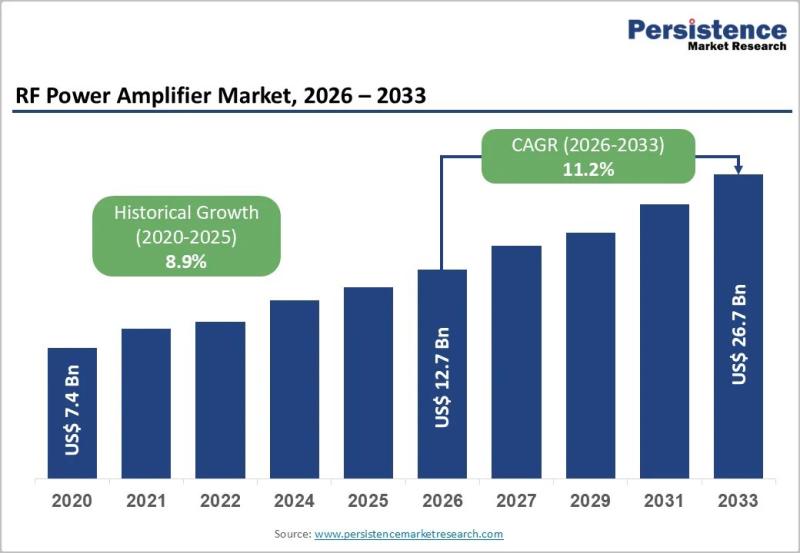
RF Power Amplifier Market Accelerates on 5G, GaN, and Defense Modernisation
RF Power Amplifier Market Overview and Growth Outlook
The global RF Power Amplifier Market is poised for sustained double-digit growth, projected to rise from US$ 12.7 billion in 2026 to US$ 26.7 billion by 2033, reflecting a strong CAGR of 11.2% during the forecast period. This expansion is underpinned by structural demand across telecommunications infrastructure, aerospace and defense modernization, and next-generation satellite communication (SATCOM) systems. As mobile data traffic surges and…
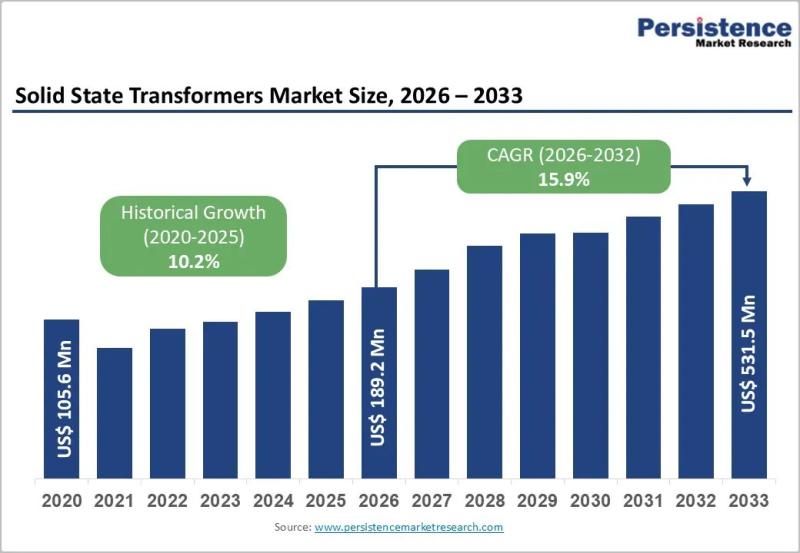
Smart Grid Expansion Driving Solid State Transformers Demand
Solid State Transformers Market Overview and Growth Outlook
The global Solid State Transformers Market is entering a high-growth phase, driven by rapid grid modernization and the accelerating shift toward renewable energy. Valued at US$ 189.2 million in 2026, the market is projected to reach US$ 531.5 million by 2033, expanding at a robust CAGR of 15.9% during the forecast period. The rising need for intelligent power distribution, bidirectional energy flow, and…
Enterprise Password Management Market to Reach US$ 9.4 Billion by 2033 as Zero-T …
The Enterprise Password Management Market is expanding rapidly as organizations confront escalating cyber threats, data breach risks, and complex regulatory mandates. Valued at US$ 3.2 billion in 2026, the market is projected to reach US$ 9.4 billion by 2033, registering a strong CAGR of 16.8% between 2026 and 2033. This accelerated growth reflects rising investment in identity security infrastructure as enterprises adopt zero-trust frameworks and strengthen credential governance.
Historically, the market…
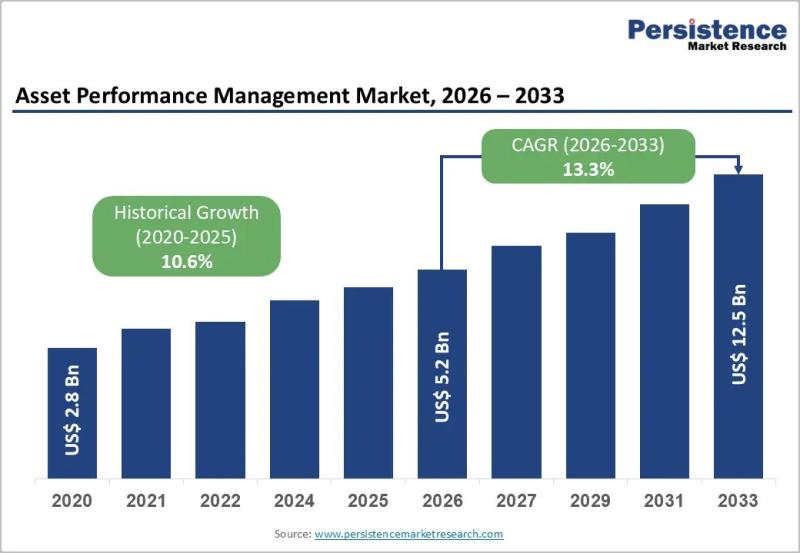
Asset Performance Management Market to Reach US$ 12.5 Billion by 2033 as AI-Driv …
Overview of the Asset Performance Management Market
The Asset Performance Management (APM) Market is witnessing strong acceleration as industries prioritize predictive maintenance, reliability engineering, and lifecycle optimization to reduce operational risks. Valued at US$ 5.2 billion in 2026, the global market is projected to reach US$ 12.5 billion by 2033, expanding at a CAGR of 13.3% between 2026 and 2033, compared to a historical CAGR of 10.6% from 2020 to 2026.…
More Releases for Cell
Cell Sorting Market Accelerates as Cell Therapy, Immuno-Oncology & Single-Cell R …
The rising focus on precision medicine, immunotherapy, and advanced cell-based research is driving the global cell sorting market into a high-growth phase. With expanding applications in stem cell therapy, CAR-T manufacturing, cancer immunology, and single-cell genomics, demand for accurate, high-purity cell isolation systems is stronger than ever. This release highlights key market trends, segmentation insights, technological innovations, and the factors shaping the future of cell sorting.
Download Full PDF Sample Copy…
Cell Isolation Cell Separation Market Size Analysis by Application, Type, and Re …
According to Market Research Intellect, the global Cell Isolation Cell Separation market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
The market for cell isolation and separation is expanding rapidly as a result of sophisticated biotechnological…
Cell Free Protein Synthesis Market Beyond the Cell: Revolutionizing Protein Prod …
Cell-Free Protein Synthesis Market to reach over USD 457.13 Mn by the year 2031 - Exclusive Report by InsightAce Analytic
"Cell-Free Protein Synthesis Market" in terms of revenue was estimated to be worth $265.94 Mn in 2023 and is poised to reach $457.13 Mn by 2031, growing at a CAGR of 7.20% from 2024 to 2031 according to a new report by InsightAce Analytic.
Request for free Sample Pages: https://www.insightaceanalytic.com/request-sample/1445
Current…
Cell Expansion Market - Expand the Boundaries of Cell Therapy: Redefine Cell Exp …
Newark, New Castle, USA: The "Cell Expansion Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Cell Expansion Market: https://www.growthplusreports.com/report/cell-expansion-market/7939
This latest report researches the industry structure, sales, revenue,…
Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect …
Researchmoz added Most up-to-date research on "Global GMP Cell Banking Market By Type - Mammalian Cell, Microbial Cell, Insect Cell and Others" to its huge collection of research reports.
This report researches the worldwide GMP Cell Banking market size (value, capacity, production and consumption) in key regions like North America, Europe, Asia Pacific (China, Japan) and other regions.
This study categorizes the global GMP Cell Banking breakdown data by manufacturers, region, type…
Cell Culture Market Size, Cell Culture Market Share, Cell Culture Market Trends …
According to a new research published by Polaris Market Research the global cell culture market is anticipated to reach more than USD 49 billion by 2026. Cell culture is a rapidly emerging as an implement for analyzing and treating various disease such as Alzheimer’s and cancer.
Request for Sample of This Research Report @ https://bit.ly/2D7pZ5u
Top Key Players: -
Becton,
Dickinson and Company
Biospherix
EMD Millipore
Eppendorf AG
Merck KGaA
Sartorius AG
VWR International
Cell culture is a rapidly emerging…
