Press release
Global Healthcare Regulatory Affairs Outsourcing Industry Poised for Strong Growth, Projected to Reach USD 4,052.30 Million by 2034
The global healthcare regulatory affairs outsourcing industry is experiencing a period of robust growth, with a projected valuation of USD 4,052.30 million by 2034. After reaching an estimated value of USD 1,985.60 million in 2023, the market is set to grow at a compound annual growth rate (CAGR) of 6.70% from 2024 to 2034. The market is expected to see a significant milestone with an anticipated value of USD 2,118.60 million by the close of 2024.As the healthcare industry continues to evolve, outsourcing has become an increasingly popular strategy for companies looking to streamline operations, reduce costs, and improve efficiency. In response, outsourcing firms are offering specialized services designed to address the growing and diverse needs of healthcare organizations. These services include providing dedicated teams of clinical research associates (CRAs), coordinators, senior monitors, and project managers on a contract basis, enabling healthcare companies to augment their internal resources without the need for extensive team expansions.
Track the Latest Market Developments: Request a Sample Report! https://www.futuremarketinsights.com/reports/sample/rep-gb-6355
"These outsourcing services are designed to offer comprehensive support to healthcare companies," said a representative from Future Market Insights. "By providing dedicated clinical experts and project management tailored to the client's needs, we ensure seamless integration and efficient project execution, allowing healthcare firms to focus on core activities while meeting regulatory requirements."
Key features of these outsourcing solutions include full-time equivalent (FTE)-based budgets with fixed and predictable pricing, ensuring financial transparency and control for healthcare companies. Additionally, outsourcing firms act as a single point of contact, managing staffing requirements while providing operational and financial oversight throughout the entire project lifecycle.
This growth trajectory highlights the crucial role the healthcare regulatory affairs outsourcing industry plays in helping healthcare companies navigate the complexities of regulatory compliance. It allows firms to optimize resource allocation, reduce costs, and accelerate the time-to-market for new therapies and medical products.
Key Takeaways from the Market Analysis:
● The global healthcare regulatory affairs outsourcing market was valued at USD 1,123.80 million in 2018.
● From 2018 to 2023, the healthcare regulatory affairs outsourcing industry experienced a CAGR of 12.10%.
● The United States healthcare regulatory affairs outsourcing industry is anticipated to develop with a 6.70% CAGR from 2024 to 2034.
● With a 33.60% market share in 2023, the regulatory writing and publishing services segment is anticipated to expand in the global healthcare regulatory affairs outsourcing industry.
● With a 29.20% market share in 2023, mid-sized pharmaceutical companies will probably be the dominant end users of healthcare regulatory affairs outsourcing.
Rising Demand for Market Data: Our Full Report Offers Deep Insights and Trend Analysis! https://www.pharmiweb.com/press-release/2024-02-07/global-healthcare-regulatory-affairs-outsourcing-industry-is-poised-to-experience-an-113-cagr-by-2
Competitive Landscape:
Market players in the global healthcare regulatory affairs outsourcing industry aim to provide pharmaceutical, biotechnology, and medical device sectors with specialized regulatory services, accelerated product approvals, compliance assurance, and process simplification.
They provide proficiency in managing intricate regulatory environments, optimizing effectiveness, and maintaining quality benchmarks in developing and promoting healthcare products.
Startups in this market are addressing regulatory obstacles for biotech, pharmaceuticals, and medical devices with customized solutions. Their primary areas of expertise include effective regulatory strategies, pharmacovigilance services, compliance monitoring, and submission preparation.
While maintaining compliance with evolving regulations, these companies want to reduce regulatory burdens, improve product development deadlines, and improve market access for advanced healthcare solutions.
Key Developments:
• In 2024, Wheeler Bio, Inc. and Charles River Laboratories International, Inc. announced a strategic partnership. Customers may now use Wheeler's innovative Portable CMC® (Chemistry, Manufacturing, and Controls) platform as a result of this partnership.
• This partnership provides early-stage biotech companies a unique route for rapidly moving from preclinical to early human clinical trial phases.
Key Companies Profiled:
• Accell Clinical Research, LLC
• Charles River Laboratories
• Syneos Health
• Laboratory Corporation of America Holdings
• ICON PLc.
• IQVIA
• Medpace, Inc.
• PAREXEL International Corporation
• Thermo Fisher Scientific Inc. (PPD)
• Promedica International
• WuXi App Tec
Key Segments Profiled in the Healthcare Regulatory Affairs Outsourcing Market:
By Services:
• Regulatory Writing and Publishing
• Regulatory Submissions
• Clinical Trial Applications
• Product Registrations
• Regulatory Consulting
• Legal Representation
By End User:
• Pharmaceutical Companies
• Biotechnology Companies
• Medical Devices Manufacturer
• Food and Beverage Companies
By Region:
• North America
• Latin America
• Western Europe
• Eastern Europe
• South Asia and Pacific
• East Asia
• Middle East & Africa
𝐄𝐱𝐩𝐥𝐨𝐫𝐞 𝐅𝐌𝐈'𝐬 𝐑𝐞𝐥𝐚𝐭𝐞𝐝 𝐎𝐧𝐠𝐨𝐢𝐧𝐠 𝐂𝐨𝐯𝐞𝐫𝐚𝐠𝐞 𝐨𝐧 𝐇𝐞𝐚𝐥𝐭𝐡𝐜𝐚𝐫𝐞 𝐌𝐚𝐫𝐤𝐞𝐭 𝐈𝐧𝐬𝐢𝐠𝐡𝐭𝐬 𝐃𝐨𝐦𝐚𝐢𝐧:
Ebstein’s Anomaly Market https://www.fmiblog.com/2024/12/10/global-ebsteins-anomaly-market-poised-for-robust-growth-projected-to-exceed-usd-30-billion-by-2033-registering-a-cagr-of-8/
Kounis Syndrome Market https://www.fmiblog.com/2024/12/10/global-kounis-syndrome-market-poised-for-remarkable-growth-projected-to-reach-usd-15990-million-by-2033-registering-a-cagr-of-5-8/
Babesiosis Treatment Market https://www.fmiblog.com/2024/12/11/global-babesiosis-treatment-market-set-to-reach-usd-1-98-billion-by-2033-fueled-by-rising-incidence-and-ongoing-pharmaceutical-advancements-fmi/
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Global Healthcare Regulatory Affairs Outsourcing Industry Poised for Strong Growth, Projected to Reach USD 4,052.30 Million by 2034 here
News-ID: 3798707 • Views: …
More Releases from Future Market Insights Inc
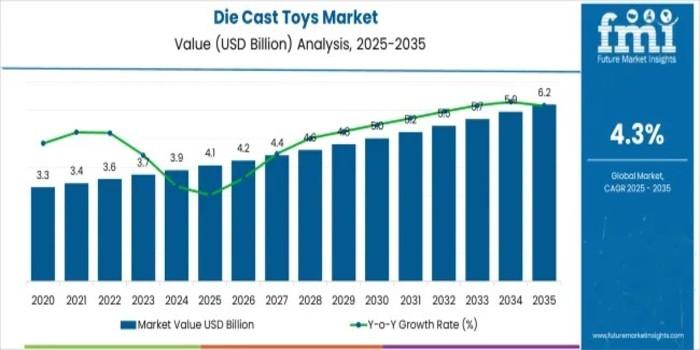
Global Die Cast Toys Market Forecast to Reach USD 6.2 Billion by 2035 Driven by …
The global die cast toys market is forecasted to expand from an estimated USD 4.1 billion in 2025 to USD 6.2 billion by 2035, exhibiting a steady compound annual growth rate (CAGR) of 4.3% over the next decade, according to the latest market analysis. This growth reflects a sustained consumer interest in collectible, high-quality die cast toys, particularly in niche markets targeting both children and adult hobbyists.
Between 2025 and 2030,…
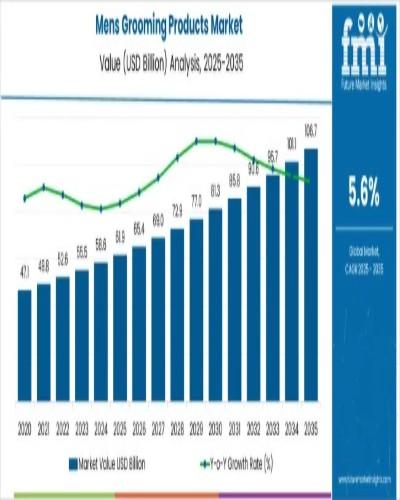
Global Men's Grooming Products Market Forecast to Reach USD 106.7 billion by 203 …
The global men's grooming products market is poised for substantial growth over the next decade, with its valuation expected to rise from USD 61.9 billion in 2025 to USD 106.7 billion by 2035. This growth reflects a robust compound annual growth rate (CAGR) of 5.6%, driven by shifting cultural norms, increased disposable incomes, and heightened consumer awareness of grooming and personal care.
The men's grooming market is set to cross two…
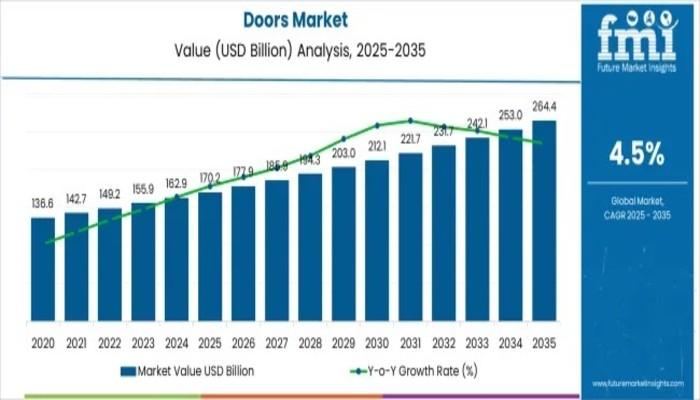
Doors Market Forecast Projects Steady 4.5% CAGR to USD 264.4 Billion by 2035 Ami …
The global doors market is poised for sustained growth, with a projected increase from USD 170.2 billion in 2025 to USD 264.4 billion by 2035, according to the latest market analysis. Registering a compound annual growth rate (CAGR) of 4.5%, the industry reflects stable demand across residential, commercial, and institutional sectors worldwide. This forecast underscores consistent procurement trends shaped by urban expansion, evolving building regulations, and advances in material and…
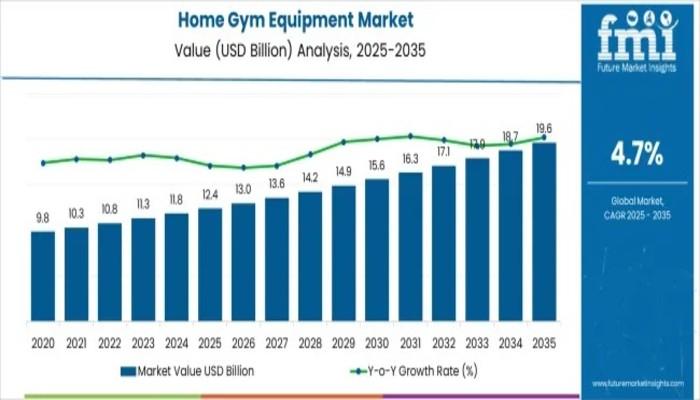
Home Gym Equipment Market Set to Reach USD 19.6 billion by 2035 Amid Rising Heal …
The Home Gym Equipment Market is forecasted to grow from an estimated value of USD 12.4 billion in 2025 to USD 19.6 billion by 2035, exhibiting a steady compound annual growth rate (CAGR) of 4.7%, according to the latest market analysis. This decade-long growth reflects evolving consumer preferences towards health and fitness, accelerated adoption of home-based workout solutions, and continuous innovation in smart fitness technologies.
Market Overview:
The global market for…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…
