Press release
Cell and Gene Therapies in Rare Disorders Market to Reach New Heights in Growth by 2034, DelveInsight Predicts | Pfizer, Sangamo Therapeutics, Orchard Therapeutics, SR-Tiget, Novartis, TVAX Biomedical
DelveInsight's "Cell and Gene Therapies in Rare Disorders Market Insights, Epidemiology, and Market Forecast-2034″ report offers an in-depth understanding of the Cell and Gene Therapies in Rare Disorders, historical and forecasted epidemiology as well as the Cell and Gene Therapies in Rare Disorders market trends in the United States, EU4 (Germany, Spain, Italy, France) the United Kingdom and Japan.To Know in detail about the Cell and Gene Therapies in Rare Disorders market outlook, drug uptake, treatment scenario and epidemiology trends, Click here; Cell and Gene Therapies in Rare Disorders Market Forecast
https://www.delveinsight.com/sample-request/gene-and-cell-therapies-in-rare-disorder-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Some of the key facts of the Cell and Gene Therapies in Rare Disorders Market Report:
• The Cell and Gene Therapies in Rare Disorders market size was valued ~USD 2,000 million in 2023 and is anticipated to grow with a significant CAGR during the study period (2020-2034)
• In 2023, the market size for cell and gene therapies targeting rare disorders in the US was around USD 800 million and is expected to grow throughout the forecast period (2024-2034).
• By 2034, Hemophilia A is projected to generate the highest revenue among all indications in the US, followed by Retinitis Pigmentosa.
• In 2023, ZOLGENSMA (onasemnogene abeparvovec-xioi) held the largest market share among therapies in the US.
• In 2023, the total prevalent cases of selected indications for Cell and Gene Therapies in Rare Disorders across the 7MM were approximately 930,200, with projections indicating an increase during the forecast period.
• In 2023, Retinitis Pigmentosa accounted for the highest number of cases among the rare indications selected for Cell and Gene Therapies, followed by Limbal Stem Cell Deficiency.
• In 2023, the total treated cases for cell and gene therapies across selected indications in the 7MM were approximately 504,000, with numbers projected to rise throughout the forecast period (2024-2034).
• Key Cell and Gene Therapies in Rare Disorders Companies: Pfizer, Sangamo Therapeutics, Orchard Therapeutics/ SR-Tiget, Novartis, TVAX Biomedical, Aivita Biomedical, RHEACELL GmbH & Co, Capricor Therapeutics, and others
• Key Cell and Gene Therapies in Rare Disorders Therapies: Fidanacogene elaparvovec, GiroctocogenE fitelparvovec, OTL-103, OAV101, TVI-Brain-1, AV-GBM-1, ABCB5+ MSCs, CAP-1002, and others
• The Cell and Gene Therapies in Rare Disorders market is expected to surge due to the disease's increasing prevalence and awareness during the forecast period. Furthermore, launching various multiple-stage Cell and Gene Therapies in Rare Disorders pipeline products will significantly revolutionize the Cell and Gene Therapies in Rare Disorders market dynamics.
Cell and Gene Therapies in Rare Disorders Overview
Cell and gene therapies are cutting-edge medical technologies with the potential to significantly improve patient care and society at large. New developments in these innovative medicines have the potential to revolutionise medicine and the ability to treat a wide range of incurable diseases.
Get a Free sample for the Cell and Gene Therapies in Rare Disorders Market Report
https://www.delveinsight.com/report-store/gene-and-cell-therapies-in-rare-disorder-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Cell and Gene Therapies in Rare Disorders Epidemiology
The epidemiology section provides insights into the historical, current, and forecasted epidemiology trends in the seven major countries (7MM) from 2020 to 2034. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. The epidemiology section also provides a detailed analysis of the diagnosed patient pool and future trends.
Cell and Gene Therapies in Rare Disorders Epidemiology Segmentation:
The Cell and Gene Therapies in Rare Disorders market report proffers epidemiological analysis for the study period 2020-2034 in the 7MM segmented into:
• Total Prevalence of Cell and Gene Therapies in Rare Disorders
• Prevalent Cases of Cell and Gene Therapies in Rare Disorders by severity
• Gender-specific Prevalence of Cell and Gene Therapies in Rare Disorders
• Diagnosed Cases of Episodic and Chronic Cell and Gene Therapies in Rare Disorders
Download the report to understand which factors are driving Cell and Gene Therapies in Rare Disorders epidemiology trends @ Cell and Gene Therapies in Rare Disorders Epidemiology Forecast
https://www.delveinsight.com/sample-request/gene-and-cell-therapies-in-rare-disorder-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Cell and Gene Therapies in Rare Disorders Drugs Uptake and Pipeline Development Activities
The drugs uptake section focuses on the rate of uptake of the potential drugs recently launched in the Cell and Gene Therapies in Rare Disorders market or expected to get launched during the study period. The analysis covers Cell and Gene Therapies in Rare Disorders market uptake by drugs, patient uptake by therapies, and sales of each drug.
Moreover, the therapeutics assessment section helps understand the drugs with the most rapid uptake and the reasons behind the maximal use of the drugs. Additionally, it compares the drugs based on market share.
The report also covers the Cell and Gene Therapies in Rare Disorders Pipeline Development Activities. It provides valuable insights about different therapeutic candidates in various stages and the key companies involved in developing targeted therapeutics. It also analyzes recent developments such as collaborations, acquisitions, mergers, licensing patent details, and other information for emerging therapies.
Cell and Gene Therapies in Rare Disorders Therapies and Key Companies
• Fidanacogene elaparvovec: Pfizer
• GiroctocogenE fitelparvovec: Pfizer/ Sangamo Therapeutics
• OTL-103: Orchard Therapeutics/ SR-Tiget
• OAV101: Novartis
• TVI-Brain-1: TVAX Biomedical
• AV-GBM-1: Aivita Biomedical
• ABCB5+ MSCs: RHEACELL GmbH & Co
• CAP-1002: Capricor Therapeutics
Discover more about therapies set to grab major Cell and Gene Therapies in Rare Disorders market share @ Cell and Gene Therapies in Rare Disorders Treatment Market
https://www.delveinsight.com/sample-request/gene-and-cell-therapies-in-rare-disorder-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Scope of the Cell and Gene Therapies in Rare Disorders Market Report
• Study Period: 2020-2034
• Coverage: 7MM [The United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan]
• Key Cell and Gene Therapies in Rare Disorders Companies: Pfizer, Sangamo Therapeutics, Orchard Therapeutics/ SR-Tiget, Novartis, TVAX Biomedical, Aivita Biomedical, RHEACELL GmbH & Co, Capricor Therapeutics, and others
• Key Cell and Gene Therapies in Rare Disorders Therapies: Fidanacogene elaparvovec, GiroctocogenE fitelparvovec, OTL-103, OAV101, TVI-Brain-1, AV-GBM-1, ABCB5+ MSCs, CAP-1002, and others
• Cell and Gene Therapies in Rare Disorders Therapeutic Assessment: Cell and Gene Therapies in Rare Disorders current marketed and Cell and Gene Therapies in Rare Disorders emerging therapies
• Cell and Gene Therapies in Rare Disorders Market Dynamics: Cell and Gene Therapies in Rare Disorders market drivers and Cell and Gene Therapies in Rare Disorders market barriers
• Competitive Intelligence Analysis: SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies
• Cell and Gene Therapies in Rare Disorders Unmet Needs, KOL's views, Analyst's views, Cell and Gene Therapies in Rare Disorders Market Access and Reimbursement
To know more about Cell and Gene Therapies in Rare Disorders companies working in the treatment market, visit @ Cell and Gene Therapies in Rare Disorders Clinical Trials and Therapeutic Assessment
https://www.delveinsight.com/sample-request/gene-and-cell-therapies-in-rare-disorder-market?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
Table of Contents
1. Cell and Gene Therapies in Rare Disorders Market Report Introduction
2. Executive Summary for Cell and Gene Therapies in Rare Disorders
3. SWOT analysis of Cell and Gene Therapies in Rare Disorders
4. Cell and Gene Therapies in Rare Disorders Patient Share (%) Overview at a Glance
5. Cell and Gene Therapies in Rare Disorders Market Overview at a Glance
6. Cell and Gene Therapies in Rare Disorders Disease Background and Overview
7. Cell and Gene Therapies in Rare Disorders Epidemiology and Patient Population
8. Country-Specific Patient Population of Cell and Gene Therapies in Rare Disorders
9. Cell and Gene Therapies in Rare Disorders Current Treatment and Medical Practices
10. Cell and Gene Therapies in Rare Disorders Unmet Needs
11. Cell and Gene Therapies in Rare Disorders Emerging Therapies
12. Cell and Gene Therapies in Rare Disorders Market Outlook
13. Country-Wise Cell and Gene Therapies in Rare Disorders Market Analysis (2020-2034)
14. Cell and Gene Therapies in Rare Disorders Market Access and Reimbursement of Therapies
15. Cell and Gene Therapies in Rare Disorders Market Drivers
16. Cell and Gene Therapies in Rare Disorders Market Barriers
17. Cell and Gene Therapies in Rare Disorders Appendix
18. Cell and Gene Therapies in Rare Disorders Report Methodology
19. DelveInsight Capabilities
20. Disclaimer
21. About DelveInsight
Related Reports:
Cell and Gene Therapies in Rare Disorders Epidemiology https://www.delveinsight.com/report-store/gene-and-cell-therapies-in-rare-disorder-epidemiology-forecast?utm_source=openpr&utm_medium=pressrelease&utm_campaign=gpr
DelveInsight's 'Cell and Gene Therapies in Rare Disorders Epidemiology Forecast to 2032' report delivers an in-depth understanding of the disease, historical and forecasted Cell and Gene Therapies in Rare Disorders epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Latest Reports by DelveInsight
• Vascular Closure Devices Market: https://www.delveinsight.com/report-store/vascular-closure-devices-market
• Apheresis Market: https://www.delveinsight.com/report-store/apheresis-market
• Hydrocephalus Market: https://www.delveinsight.com/report-store/hydrocephalus-market
• Hemophilia A Market: https://www.delveinsight.com/report-store/hemophilia-a2030-market
• Herpes Simplex Virus Market: https://www.delveinsight.com/report-store/genital-herpes-market
• Peripheral Nerve Injuries Market: https://www.delveinsight.com/infographics/peripheral-nerve-injuries-market
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Gaurav Bora
Email: info@delveinsight.com
City: Albany
State: New York
Country: United States
Website: https://www.delveinsight.com/consulting
About DelveInsight
DelveInsight is a leading Healthcare Business Consultant, and Market Research firm focused exclusively on life sciences. It supports Pharma companies by providing comprehensive end-to-end solutions to improve their performance.
It also offers Healthcare Consulting Services, which benefits in market analysis to accelerate the business growth and overcome challenges with a practical approach.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Cell and Gene Therapies in Rare Disorders Market to Reach New Heights in Growth by 2034, DelveInsight Predicts | Pfizer, Sangamo Therapeutics, Orchard Therapeutics, SR-Tiget, Novartis, TVAX Biomedical here
News-ID: 3781747 • Views: …
More Releases from DelveInsight Business Research
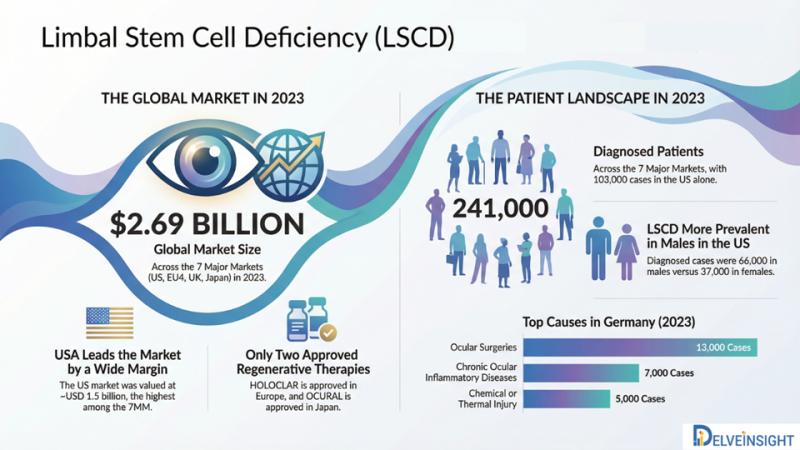
Limbal Stem Cell Deficiency Market to Surpass USD 2.6 Billion by 2034, Driven by …
In 2023, the Limbal Stem Cell Deficiency (LSCD) market was dominated by the United States, generating nearly USD 1.5 billion in revenue, while Spain represented the smallest market with approximately USD 127 million. This regional distribution is expected to remain consistent throughout the forecast timeline. The US accounted for nearly 103,000 diagnosed LSCD cases, whereas Japan recorded around 37,000 cases, with both countries projected to witness notable growth in patient…
SSc-ILD Market Set to Cross USD 750 Million by 2034, Driven by 10+ Emerging Ther …
The major players operating in the Systemic Sclerosis-associated Interstitial Lung Disease (SSc-ILD) market include Roche, Prometheus Biosciences, Inc., Merck, GlaxoSmithKline, Genentech, Inc., Acceleron, Boehringer Ingelheim, Actelion, Hôpital Claude-Huriez, Changchun GeneScience Pharmaceutical, among others.
DelveInsight's report titled "Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Forecast-2034" delivers a comprehensive analysis of SSc-ILD, covering historical data, projected epidemiology, and evolving market trends across the United States, EU4 (Germany, Spain, Italy, and France),…
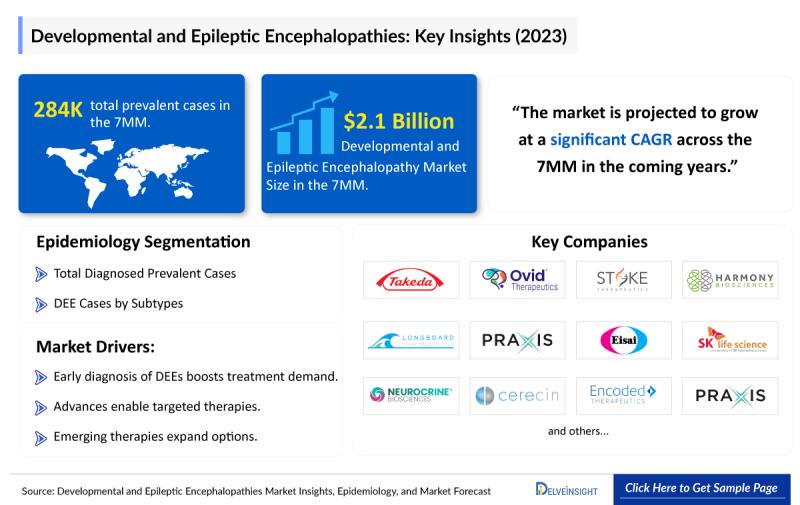
Developmental and Epileptic Encephalopathy Treatment Market Poised for Accelerat …
The Developmental and Epileptic Encephalopathy (DEE) treatment market across the seven major markets (7MM) was valued at nearly USD 2.1 billion in 2023 and is expected to register a healthy compound annual growth rate over the forecast period. The United States emerged as the largest contributor, capturing close to 80% of the overall market revenue.
The DEE treatment landscape is undergoing a significant transformation as the limitations of traditional antiepileptic drugs…
Polycythemia Vera Pipeline and Drug Development in 2025: 10+ Therapies and 8+ Co …
DelveInsight's "Polycythemia Vera Pipeline Insight 2025" report delivers an in-depth overview of the Polycythemia Vera pipeline landscape, covering more than 8 companies and 10+ pipeline candidates. The report analyzes both clinical-stage and preclinical assets, offering detailed drug profiles across various stages of development. It also evaluates Polycythemia Vera therapies based on product classification, development stage, route of administration, and molecular category, while additionally spotlighting inactive or discontinued pipeline assets.
Explore the…
More Releases for Therapies
GM1 Gangliosidosis Market: Epidemiology, Therapies, Companies, DelveInsight | Pa …
GM1 Gangliosidosis therapies, such as PBGM01, and others, are expected to boost the GM1 Gangliosidosis Market in the upcoming years.
DelveInsight has launched a new report on "GM1 Gangliosidosis - Market Insights, Epidemiology, and Market Forecast-2034" that delivers an in-depth understanding of the GM1 Gangliosidosis, historical and forecasted epidemiology as well as the GM1 Gangliosidosis market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
Discover…
Facioscapulohumeral Muscular Dystrophy Pipeline Assessment 2024: Therapies, Clin …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Facioscapulohumeral Muscular Dystrophy pipeline constitutes 10+ key companies continuously working towards developing 10+ Facioscapulohumeral Muscular Dystrophy treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"Facioscapulohumeral Muscular Dystrophy Pipeline Insight, 2023" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Facioscapulohumeral Muscular…
Intratumoral Cancer Therapies Market Report 2024 - Intratumoral Cancer Therapies …
"The Business Research Company recently released a comprehensive report on the Global Intratumoral Cancer Therapies Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
According to The Business Research Company's, The intratumoral cancer therapies…
Innovative Therapies in Alcohol Rehabilitation
Alcohol rehabilitation has evolved significantly over the years, with innovative therapies emerging as crucial elements in the journey to recovery. These groundbreaking approaches go beyond traditional methods, providing individuals struggling with alcohol addiction with a diverse set of tools to achieve and maintain sobriety. In this article, we delve into innovative therapies, exploring their effectiveness and impact on alcohol rehabilitation if you'd like to know more about alcohol rehab in…
B-cell Maturation Antigen (BCMA) Targeted Therapies Market New Development of No …
A global prevalence of over one million cases. B-cell maturation antigen (BCMA), is a cell surface protein that is expressed on the malignant plasma cells. This cell surface protein has emerged as a very selective antigen targeted therapy for the treatment of multiple myeloma. BCMA targeted therapies actively involve three major types of immunotherapies on the basis of product class namely, chimeric antigen receptor T-cells (CAR T Cells), bispecific antibodies,…
Viral Vector Contract Manufacturing Market Outlook 2021- Gene Therapies, Cell Th …
The latest report released on Outlook for Viral Vector Contract Manufacturing - Gene Therapies, Cell Therapies, and COVID-19 Vaccines Market analyses areas where there is still room for improvement. Irrespective of industry, organization size or geographic location, the Outlook for Viral Vector Contract Manufacturing - Gene Therapies, Cell Therapies, and COVID-19 Vaccines Market study suggests that advanced technologies are playing a bigger role than ever before. The assessment provides trend,…
