Press release
Clinical Trial Biorepository & Archiving Solutions Market Key Players Analysis - Azenta U.S., Inc., Thermo Fisher Scientific Inc. (Patheon), Precision for Medicine, Inc., Medpace.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Biorepository & Archiving Solutions Market- (By Service (Archiving Solution, Biorepository Services), By Product (Preclinical, Clinical), By Phase (I, II, III,IV)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."According to the latest research by InsightAce Analytic, the Global Clinical Trial Biorepository & Archiving Solutions Market is valued at US$ 3.98 Bn in 2023, and it is expected to reach US$ 7.73 Bn by 2031, with a CAGR of 8.82% during the forecast period of 2024-2031.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2553
Clinical Trial Biorepository & Archiving Solutions are essential infrastructures that support the integrity and efficiency of clinical trials, crucial in evaluating the safety and efficacy of new medical interventions. These solutions encompass specialized facilities and technologies dedicated to storing, managing, and preserving biological samples and clinical trial data. Biorepository services form a cornerstone by providing secure environments for the storage of biological samples such as blood, tissue, and bodily fluids collected during trials. These facilities ensure samples are meticulously handled, maintained, and documented to uphold their integrity and regulatory compliance, essential for subsequent research and analysis.
Archiving solution services are crucial for managing the extensive data produced during clinical trials. These services systematically organize and preserve data, ensuring compliance with regulatory standards while facilitating easy access to critical trial information. Leveraging advanced data management systems, archiving solutions foster collaboration among researchers and pharmaceutical firms, enabling thorough analysis and generating valuable insights that drive medical progress. Overall, Clinical Trial Biorepository & Archiving Solutions constitute vital infrastructure that upholds rigorous standards and promotes collaborative efforts crucial for advancing healthcare research and development.
List of Prominent Players in the Clinical Trial Biorepository & Archiving Solutions Market:
• Azenta U.S., Inc.
• Thermo Fisher Scientific Inc. (Patheon)
• Precision for Medicine, Inc.
• Medpace
• LabCorp Drug Development
• ATCC
• Q2 Solutions
• Labconnect
• Charles River Laboratories
• Cell&Co
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2024-02
Market Dynamics:
Drivers:
The Clinical Trial Biorepository & Archiving Solutions market is experiencing rapid growth driven by several key factors. There is an increasing demand for biorepositories to securely store and manage biological samples and clinical trial data, supporting the efficient conduct of research and development activities. Advancements in biorepository technology, such as automated sample handling, cryopreservation improvements, and enhanced sample tracking systems, are enhancing operational efficiency and effectiveness. Moreover, the growing emphasis on personalized medicine is fueling demand for biorepository and archiving solutions, crucial for developing targeted therapies. Rising research and development activities, coupled with government support through funding initiatives, are further propelling market expansion. These solutions also facilitate collaboration among researchers and pharmaceutical companies, enabling the discovery of biomarkers and the development of innovative therapeutics, while advanced data management systems streamline integration and analysis of sample data, fostering research collaboration and driving data-driven insights in healthcare research and development efforts.
Challenges:
The Clinical Trial Biorepository & Archiving Solutions market faces several significant challenges based on current insights. Regulatory complexity stands out, as maintaining compliance with stringent regulations like HIPAA, FDA guidelines, and various global standards for sample storage, data management, and patient privacy proves demanding. Coordinating and standardizing biorepository procedures across multiple sites adds to the complexity. High costs associated with infrastructure such as refrigeration, temperature monitoring, inventory management, and equipment maintenance present financial hurdles, particularly for smaller pharmaceutical companies that may opt for outsourcing.
Regional Trends:
North America holds a leading position in the Clinical Trial Biorepository & Archiving Solutions market. With 27 percent of all global clinical drug trials initiated in 2021 originating in North America, the region showcases its pivotal role in pharmaceutical research and development. This leadership is bolstered by a dense network of pharmaceutical companies and research institutions that conduct extensive clinical trials, driving significant demand for biorepository and archiving solutions. These solutions are critical for managing and securely storing biological samples and clinical trial data, essential for advancing medical research and ensuring regulatory compliance. North America's robust investment in R&D further underscores its dominance in driving innovation and therapeutic development through advanced biorepository and archiving infrastructure
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2553
Recent Developments:
• In June 2024, Labcorp, a prominent provider of extensive laboratory services, has unveiled Labcorp Global Trial Connect, a suite of central laboratory solutions designed to expedite clinical trials conducted at investigator sites, the core of clinical research.
• In June 2024, Azenta, Inc. has been chosen by the Crohn's & Colitis Foundation to manage samples for two major research cohorts: CAPTURE IBD and IBD SIRQC. Azenta will handle sample processing, analysis, logistics, and storage for these studies focusing on pediatric and surgical care for Crohn's disease and ulcerative colitis.
Segmentation of Clinical Trial Biorepository & Archiving Solutions Market-
By Service
• Biorepository Services
o Warehousing & Storage
o Transportation
o Sample Processing
o Others
• Archiving Solution Services
o Database Indexing and Management
o Scanning & Destruction
By Product
• Preclinical Products
• Clinical Products
o Human Tissue
o Organs
o Stem Cells
o Other Biospecimens
By Phase
• Phase I
• Phase II
• Phase III
• Phase IV
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Empower Your Decision-Making with 180 Pages Full Report @ https://www.insightaceanalytic.com/buy-report/2553
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
Contact us:
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
info@insightaceanalytic.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Biorepository & Archiving Solutions Market Key Players Analysis - Azenta U.S., Inc., Thermo Fisher Scientific Inc. (Patheon), Precision for Medicine, Inc., Medpace. here
News-ID: 3775286 • Views: …
More Releases from InsightAce Analytic Pvt. Ltd
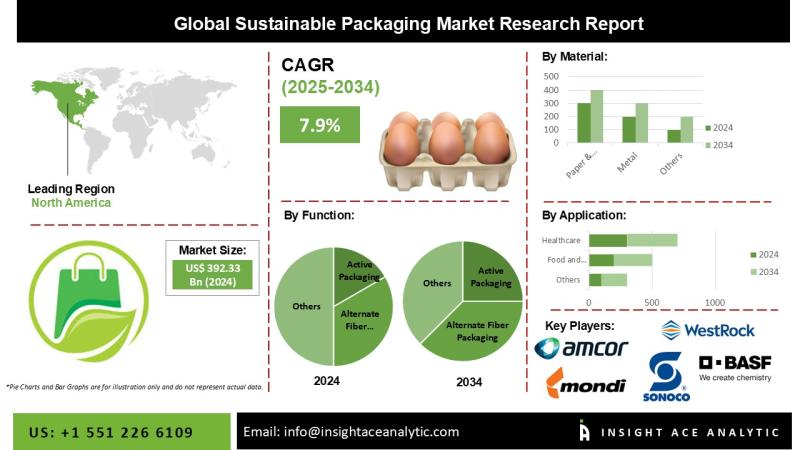
Sustainable Packaging Market Strategic Industry Overview and Forecast 2026 to 20 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Sustainable Packaging Market Size, Share & Trends Analysis Report By Material (Paper & Paperboard, Plastic, Metal, Glass), Process (Recycled, Reusable, Degradable), Function (Active, Molded Pulp, Alternate Fiber), Application (Food & Beverage, Healthcare, Others) & Layer (primary, secondary, and tertiary)- Market Outlook And Industry Analysis 2034"
The global Sustainable Packaging Market is estimated to reach over USD…
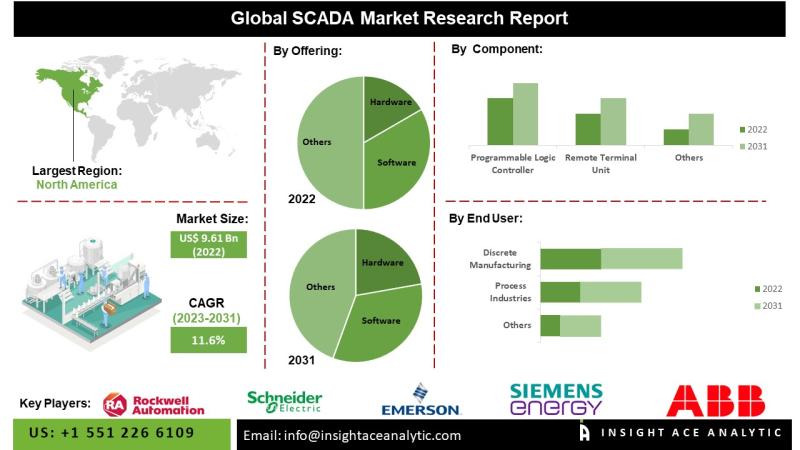
SCADA Market Insights Highlighting Technological Advancements in Wireless Sensor …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global SCADA Market Size, Share & Trends Analysis Report By Offering (Hardware, Software, Services), Component (Programmable Logic Controller, Remote Terminal Unit, Human-Machine Interface), End User (Process Industries, Discrete Manufacturing, Utilities), Region, Market Outlook And Industry Analysis 2034"
The global SCADA market is estimated to reach over USD 25.0 billion by the year 2034, exhibiting a CAGR of…
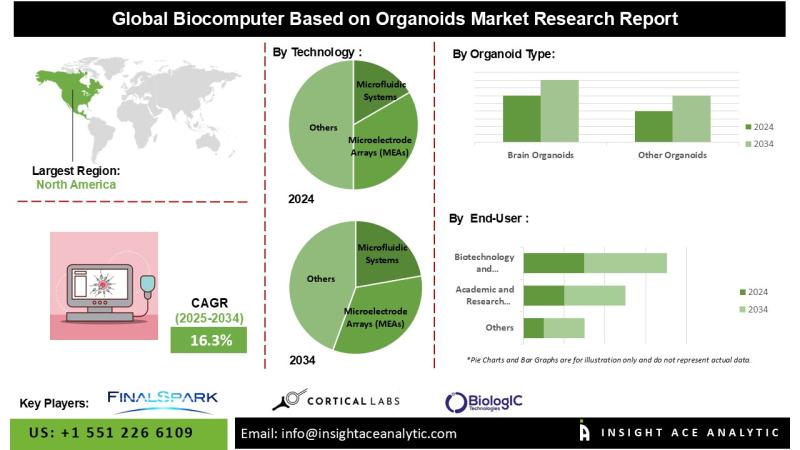
Biocomputer Based on Organoids Market Poised for 16.3% CAGR Driven by Brain Orga …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Biocomputer Based on Organoids Market Size, Share & Trends Analysis Report By Organoid Type (Brain Organoids, Other Organoids), Application (Biological Computing, Neuroscience Research, Drug Discovery and Testing, Personalized Medicine, Regenerative Medicine), Technology (Microfluidic Systems Microelectrode Arrays (MEAs), Brain-Machine Interfaces, CRISPR and Gene Editing), End-User (Academic and Research Institutes, Biotechnology and Pharmaceutical Companies, Technology Companies, Contract…
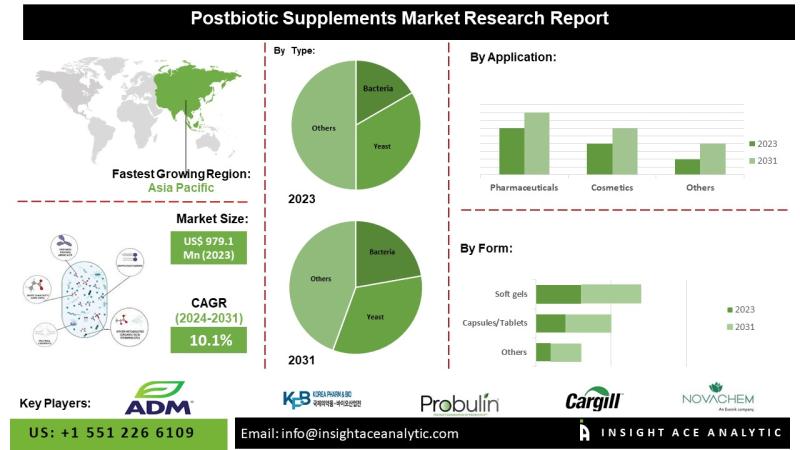
Postbiotic Supplements Market Drivers Include Functional Nutrition and Bioactive …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Postbiotic Supplements Market - (By Type (Bacteria, Yeast), By Form (Soft gels, Capsules/Tablets, Powder/ Granules, Others), By Application (Personal Care and Cosmetics, Food and Beverages, Animal Feed, Pharmaceuticals, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Postbiotic Supplements Market is valued at USD 12.8…
More Releases for Biorepository
Biorepository Service Market is Booming Worldwide | Major Giants Thermo Fisher S …
HTF MI just released the Global Biorepository Service Market Study, a comprehensive analysis of the market that spans more than 143+ pages and describes the product and industry scope as well as the market prognosis and status for 2025-2033. The marketization process is being accelerated by the market study's segmentation by important regions. The market is currently expanding its reach.
Major Manufacturers are covered: Thermo Fisher Scientific (United States), Brooks Life…
Clinical Trial Biorepository & Archiving Solutions Market Growth Set to Surge Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Biorepository & Archiving Solutions Market- (By Service (Archiving Solution, Biorepository Services), By Product (Preclinical, Clinical), By Phase (I, II, III,IV)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Biorepository & Archiving Solutions Market is valued at US$ 3.98 Bn…
Clinical Trial Biorepository & Archiving Solutions Market Robust Expansion is ex …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Biorepository & Archiving Solutions Market- (By Service (Archiving Solution, Biorepository Services), By Product (Preclinical, Clinical), By Phase (I, II, III,IV)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Biorepository & Archiving Solutions Market is valued at US$ 3.98 Bn…
Clinical Trial Biorepository and Archiving Solutions Market Rising as Regulatory …
Clinical Trial Biorepository and Archiving Solutions Market Overview
The Clinical Trial Biorepository and Archiving Solutions Market is estimated to be valued at USD 4.98 Bn in 2025 and is expected to reach USD 9.96 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.4% from 2025 to 2032.
Coherent Market Insights has published a detailed report offering a comprehensive analysis of the Clinical Trial Biorepository and Archiving Solutions Market for…
Global Clinical Trial Biorepository & Archiving Solutions Market Exclusive Repor …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Biorepository & Archiving Solutions Market- (By Service (Archiving Solution, Biorepository Services), By Product (Preclinical, Clinical), By Phase (I, II, III,IV)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Biorepository & Archiving Solutions Market is valued at US$ 3.98 Bn…
Clinical Trial Biorepository & Archiving Solutions Market Vendor and Technology …
Global Clinical Trial Biorepository & Archiving Solutions Market worth $7.73 Bn by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Biorepository & Archiving Solutions Market- (By Service (Archiving Solution, Biorepository Services), By Product (Preclinical, Clinical), By Phase (I, II, III,IV)), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
Get Free Access to Demo…
