Press release
BioEnable Technologies Achieves MOSIP Compliance for Secure, Portable Biometric Registration Kit
Image: https://www.abnewswire.com/uploads/04d8b6199818350284d1d330f17b836b.jpgNov 28, 2024 - Pune, India - BioEnable Technologies Pvt Ltd [https://www.bioenabletech.com/] has earned MOSIP compliance for its Biometric Registration Kit, empowering organizations worldwide to securely establish mobile Identity Registration Centers in a variety of locations. This certification marks a significant step in BioEnable's mission to provide robust, mobile identity solutions that meet the highest standards of data security and interoperability.
Designed for flexibility and durability, the BioEnable Biometric Registration Kit [https://www.bioenabletech.com/products/bioenable-biometric-registration-kit] includes a 4-4-2 fingerprint scanner, Dual iris scanner, face capture camera, document scanner, and a printer, providing a full suite of tools for rapid identity capture and verification. Built into a rugged IP67-certified case and paired with an advanced battery management system, the kit is protected for deployment in challenging conditions while offering continuous operation throughout the day.
This MOSIP-compliant device enables BioEnable to extend its reach into new markets where secure and portable identity registration is essential for government programs, financial services, law enforcement, and healthcare. With MOSIP's modular approach to identity systems, BioEnable's Biometric Registration Kit [https://www.bioenabletech.com/products/bioenable-biometric-registration-kit] integrates seamlessly with diverse ecosystems, allowing for secure digital identities without vendor lock-in.
Key Features of the BioEnable Biometric Registration Kit:
* 4-4-2 Fingerprint Scanner: Ensures high accuracy in capturing fingerprints.
* Dual Iris Scanner: high quality iris captures through advanced Dual Iris scanner
* Face Capture Camera & Document Scanner: Completes a holistic identity capture suite.
* Rugged IP67-Certified Case: Protects equipment in extreme conditions.
* Advanced Battery Management System: Provides reliable, full-day operation even in remote areas.
"At BioEnable, we are committed to meeting the evolving needs of our clients with adaptable, high-quality solutions," said Mr. Mahesh Ghatge, CEO and CTO of BioEnable Technologies. "Our MOSIP-compliant Biometric Registration Kit reflects BioEnable's commitment to pioneering secure, adaptable identity solutions for clients around the world. This certification highlights our dedication to setting new standards in secure identity registration for critical applications across sectors."
The BioEnable Biometric Registration Kit is ideal for:
- Government programs and Voter Registration: providing secure, scalable citizen identity solutions crucial for fair and transparent elections.
- Border Control and Immigration: enabling efficient identity verification at borders, ensuring secure and streamlined processing for immigration and customs.
- Financial Services: enhancing KYC (Know Your Customer) processes to improve security and compliance in banking and financial transactions.
- Law Enforcement: supporting portable, reliable identity verification tools in diverse and remote environments.
- Healthcare: enabling efficient patient registration in rural areas and mobile clinics, ensuring identity verification for secure healthcare delivery
About BioEnable Technologies
A pioneer in Identification, Automation, and Tracking solutions, BioEnable Technologies operates across three continents, collaborating with multinational clients to deliver complex and mission-critical technology solutions. Known for reliability and a strong ethical approach, BioEnable offers expertise and global reach in secure identity and data management. With this MOSIP-compliant kit, BioEnable continues to drive innovation in secure identity registration technology, building a foundation for trusted digital identities globally.
For more information, please visit www.bioenabletech.com [http://www.bioenabletech.com/].
Image: https://www.abnewswire.com/uploads/1d3217099ccab57f8a1f7c6449085f02.jpg
Media Contact
Company Name: BioEnable Technologies
Contact Person: Mahesh Ghatge
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=bioenable-technologies-achieves-mosip-compliance-for-secure-portable-biometric-registration-kit]
Country: India
Website: https://www.bioenabletech.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release BioEnable Technologies Achieves MOSIP Compliance for Secure, Portable Biometric Registration Kit here
News-ID: 3762612 • Views: …
More Releases from ABNewswire
Best Creative Director Side Projects of 2026 Demonstrate AI Integration and Cult …
How Side Projects Became the New Portfolio for Creative Leadership Roles
SEATTLE, WA - February 6, 2026 - The best creative director side projects are no longer optional-they're essential signals of leadership readiness. As AI transforms advertising workflows, creative directors who demonstrate hands-on technical curiosity through side projects are landing senior roles at 3x the rate of those who rely solely on client work portfolios, according to creative recruiting data.
"The resume…

IgA Nephropathy Pipeline 2025: FDA Updates, Therapy Innovations, and Clinical Tr …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, IgA Nephropathy pipeline constitutes 25+ key companies continuously working towards developing 30+ IgA Nephropathy treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"IgA Nephropathy Pipeline Insight, 2025" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the IgA Nephropathy Market.
The IgA Nephropathy Pipeline report embraces…
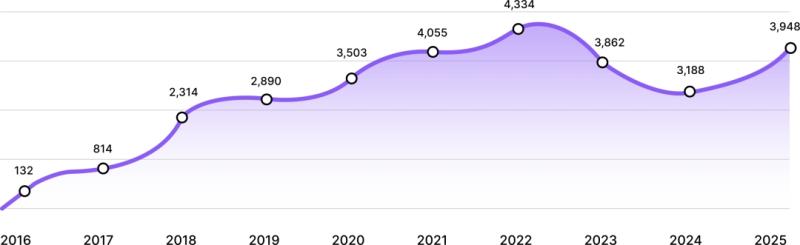
EcomBack Releases 2025 Website Accessibility Lawsuits Report: 3,948 Businesses F …
EcomBack's 2025 ADA Website Accessibility Lawsuit Report reveals nearly 4,000 lawsuits, key industry targets, and trends in plaintiff and law firm activity
Los Angeles, CA - EcomBack, a leading digital accessibility company, has released its 2025 ADA Website Accessibility Lawsuit Report [https://www.ecomback.com/annual-2025-ada-website-accessibility-lawsuit-report], revealing a continued surge in legal actions against businesses over website accessibility.
The report documents 3,948 ADA-related website lawsuits filed in 2025, highlighting the urgent need for companies to prioritize…

Discount Bad Omens Do You Feel Love 2026 Tickets + Beartooth & President - Promo …
Get discounted 2026 Bad Omens Do You Feel Love tour tickets with Beartooth & President! Use promo code CITY10 at CapitalCityTickets.com to score cheap seats and exclusive deals. Don't miss this high-energy tour-secure premium spots, enjoy unforgettable live performances, and save big on all dates. Grab last-minute tickets and experience Bad Omens, Beartooth, and President live in 2026 at the best prices online.
Bad Omens, the platinum-certified rock/metalcore powerhouse known for…
More Releases for BioEnable
Biometric in Automotive Market is Touching New Level | BioEnable, Miaxis, Sonava …
The Latest published market study on Global Biometric in Automotive Market provides an overview of the current market dynamics in the Biometric in Automotive space, as well as what our survey respondents all outsourcing decision-makers predict the market will look like in 2029. The study breaks the market by revenue and volume (wherever applicable) and price history to estimate the size and trend analysis and identify gaps and opportunities. Some…
Fingerprint Biometrics Market to Witness Huge Growth by 2028 | BioEnable, Liteon …
Worldwide Fingerprint Biometrics Market In-depth Research Report 2022, Forecast to 2029 is the latest research study released by HTF MI evaluating the market risk side analysis, highlighting opportunities, and leveraging with strategic and tactical decision-making support. The report provides information on market trends and development, growth drivers, technologies, and the changing investment structure of the Worldwide Fingerprint Biometrics Market. Some of the key players profiled in the study are Gemalto,…
Fingerprint Scanner Market Shows Tremendous Growth worldwide | 3M, BioEnable, BI …
Fingerprint Scanner Market Report Highlights The Competitive Scenario With, Size, Share, Trends, Demand, Market Dynamics, Growth, Competitors Outlook, Impact of Drivers, Challenges, and Opportunity Assessment
New Jersey, United States - The Infinity Business Insights published a new study on the Global Fingerprint Scanner Market exclusive insights, Opportunities and revenue size estimation and growth factors. The Worldwide Fingerprint Scanner market size is estimated to be worth USD million in 2022 and is forecast…
Biometric Iris Recognition System Market 2021 Top Key Manufacturers, Latest Tren …
LOS ANGELES, United States: The research study presented in this report offers complete and intelligent analysis of the competition, segmentation, dynamics, and geographical advancement of the global Biometric Iris Recognition System market. It takes into account the CAGR, value, volume, revenue, production, consumption, sales, manufacturing cost, prices, and other key factors related to the global Biometric Iris Recognition System market. The authors of the report have segmented the global Biometric…
Biometric ATM Market Booming Segments; Investors Seeking Stunning Growth | Hitac …
Advance Market Analytics recently introduced Global Biometric ATM Market study with in-depth overview, describing about the Product / Industry Scope and elaborates market outlook and status to 2025. Global Biometric ATM effective study on varied sections of Industry like opportunities, size, growth, technology, demand and trend of high leading players. It also provides market key statistics on the status of manufacturers, a valuable source of guidance, direction for companies and…
Global Palm Vein Biometric Device Market Forecast 2018-2025 BioEnable, Hitachi, …
Market study on Global Palm Vein Biometric Device 2018 Research Report presents a professional and complete analysis of Global Palm Vein Biometric Device Market on the current market situation.
Report provides a general overview of the Palm Vein Biometric Device industry 2018 including definitions, classifications, Palm Vein Biometric Device market analysis, a wide range of applications and Palm Vein Biometric Device industry chain structure. The 2018's report on Palm Vein…
