Press release
Eosinophilic Esophagitis Pipeline Therapeutics, Assessment, Companies, Products, Unmet Needs, Market Drivers and Barriers
DelveInsight's, "Eosinophilic Esophagitis- Pipeline Insight, 2024" report provides comprehensive insights about 25+ companies and 30+ pipeline drugs in Eosinophilic Esophagitis pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our latest breakthroughs in Eosinophilic Esophagitis research. Learn more about our innovative pipeline today! @ Eosinophilic Esophagitis Pipeline Outlook [https://www.delveinsight.com/sample-request/eosinophilic-esophagitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Eosinophilic Esophagitis Pipeline Report
* In October 2024:- Sanofi- This is parallel, Phase 4 study which consists of a 24 week (0.5 years) randomized, double blind, placebo controlled, 2-arm treatment period followed by an open label segment of 104 weeks (2 years) for a total of 128 weeks (2.5 years) to evaluate the effect of dupilumab treatment on esophageal function, and remodeling in adults with eosinophilic esophagitis.
* In October 2024:- AstraZeneca- The study consists of a screening period of 2 to 8 weeks and a 52-week randomized double-blind placebo-controlled treatment period. After completion of the treatment period, participants will be eligible to participate in an optional active treatment extension period (lasting for 24 weeks), followed by a 12-week off-treatment safety follow-up period. Participants who will not participate in the extension period will participate in a 12-week off-treatment safety follow-up period following completion of the 52-week treatment period.
* DelveInsight's Eosinophilic Esophagitis pipeline report depicts a robust space with 25+ active players working to develop 30+ pipeline therapies for Eosinophilic Esophagitis treatment.
* The leading Eosinophilic Esophagitis Companies such as Ellodi Pharmaceuticals, Revolo Biotherapeutics, Aqilion, Bristol-Myers Squibb, EsoCap, Pfizer, Calypso Biotech, Serpin Pharma, Landos Biopharma , and others.
* Promising Eosinophilic Esophagitis Therapies such as Dupilumab, Tezepelumab, NDX-3315, Barzolvolimab, CC-93538, EP-104IAR, Budesonide , and others.
Stay informed about the cutting-edge advancements in Eosinophilic Esophagitis treatments. Download for updates and be a part of the revolution in care @ Eosinophilic Esophagitis Clinical Trials Assessment [https://www.delveinsight.com/sample-request/eosinophilic-esophagitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Eosinophilic Esophagitis Emerging Drugs Profile
* APT-1011: Ellodi Pharmaceuticals
APT-1011 is a novel, proprietary, once daily, investigational oral disintegrating tablet designed to deliver fluticasone propionate to the esophageal mucosa while minimizing residence time in the oral cavity. In earlier clinical trials, APT-1011 reduced esophageal eosinophil counts and endoscopic findings in adults with a diagnosis of EoE. It is currently in clinical development, following successful completion of FLUTE I and FLUTE II. It has been granted orphan drug status by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Currently the product is in Phase III stage of development for the treatment of Eosinophilic Esophagitis.
* IRL201104: Revolo Biotherapeutics
IRL201104, is uniquely safe and efficacious demonstrating immunoregulatory activity in a number of preclinical models. It has successfully completed Phase I clinical development and has the potential to be the first Immune Resetting Asthma Drug (IMRAD). 1104 is a novel linear peptide, derived from a natural immune-regulatory protein, Mycobacterium tuberculosis Chaperonin 60.1, involved in resetting the immune system. It may provide remission for many allergic diseases. 1104 engages a novel target on macrophages and is a first-in-class modulator of the macrophage response. This response engages not only the effector arm of the immune system, but the regulatory arm as well. Currently the product is in Phase II stage of development for the treatment of patients with eosinophilic esophagitis (EoE) and other Th2 allergic diseases.
* AQ280: Aqilion
AQ280 is an oral, small-molecule, super selective JAK1 inhibitor. JAK1 is an enzyme, a kinase that accelerates inflammatory processes, which, among other things effects allergy and fibrosis. By inhibiting its mechanism, it is possible to reduce symptoms and disease development in chronic inflammatory diseases. Within the Regulus program, Aqilion is developing AQ280, which is a super selective JAK1 inhibitor, as a treatment of the chronic disease eosinophilic esophagitis. Data describing the pharmacokinetic profile, safety and tolerability of the drug candidate was evaluated between every dose escalation. Now, data has been collected for all dose groups and the database has been locked for statistical analysis. Currently the product is in Phase I stage of development for the treatment of patients with eosinophilic esophagitis (EoE).
Learn more about Eosinophilic Esophagitis Drugs opportunities in our groundbreaking Eosinophilic Esophagitis research and development projects @ Eosinophilic Esophagitis Unmet Needs [https://www.delveinsight.com/sample-request/eosinophilic-esophagitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Eosinophilic Esophagitis Companies
Ellodi Pharmaceuticals, Revolo Biotherapeutics, Aqilion, Bristol-Myers Squibb, EsoCap, Pfizer, Calypso Biotech, Serpin Pharma, Landos Biopharma, and others.
Eosinophilic Esophagitis pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs such as
* Intravenous
Eosinophilic Esophagitis Products have been categorized under various Molecule types such as
* Peptide
* Protein
* Propylene glycols
* Cell Therapy
Discover the latest advancements in Eosinophilic Esophagitis treatment by visiting our website. Stay informed about how we're transforming the future of Immunological and Autoimmune Disorders @ Eosinophilic Esophagitis Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/eosinophilic-esophagitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Eosinophilic Esophagitis Pipeline Report
* Coverage- Global
* Eosinophilic Esophagitis Companies- Ellodi Pharmaceuticals, Revolo Biotherapeutics, Aqilion, Bristol-Myers Squibb, EsoCap, Pfizer, Calypso Biotech, Serpin Pharma, Landos Biopharma, and others.
* Eosinophilic Esophagitis Therapies- Dupilumab, Tezepelumab, NDX-3315, Barzolvolimab, CC-93538, EP-104IAR, Budesonide, and others.
* Eosinophilic Esophagitis Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Eosinophilic Esophagitis Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
For a detailed overview of our latest research findings and future plans, read the full details of Eosinophilic Esophagitis Pipeline on our website @ Eosinophilic Esophagitis Emerging Drugs and Companies [https://www.delveinsight.com/sample-request/eosinophilic-esophagitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Eosinophilic Esophagitis: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Eosinophilic Esophagitis - DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* APT-1011: Ellodi Pharmaceuticals
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* IRL201104: Revolo Biotherapeutics
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* AQ280: Aqilion
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug Name: Company Name
* Drug profiles in the detailed report.....
* Inactive Products
* Eosinophilic Esophagitis Key Companies
* Eosinophilic Esophagitis Key Products
* Eosinophilic Esophagitis- Unmet Needs
* Eosinophilic Esophagitis- Market Drivers and Barriers
* Eosinophilic Esophagitis- Future Perspectives and Conclusion
* Eosinophilic Esophagitis Analyst Views
* Eosinophilic Esophagitis Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: yash bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=eosinophilic-esophagitis-pipeline-therapeutics-assessment-companies-products-unmet-needs-market-drivers-and-barriers]
Phone: 09650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Eosinophilic Esophagitis Pipeline Therapeutics, Assessment, Companies, Products, Unmet Needs, Market Drivers and Barriers here
News-ID: 3714781 • Views: …
More Releases from ABNewswire
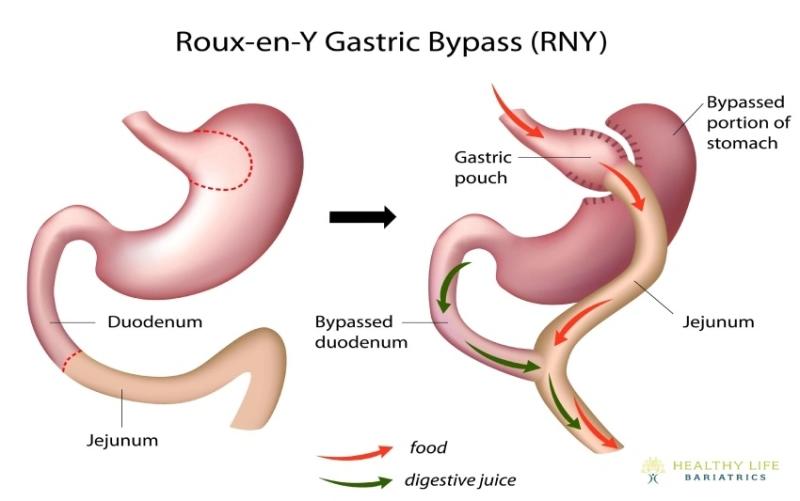
Gastric Bypass Revision: Evidence-Based Indications and Techniques
Gastric bypass revision is a corrective intervention for post-Roux-en-Y patients with weight regain, inadequate weight loss, or procedure-related complications. Using endoscopic (e.g., outlet reduction) or minimally invasive surgical techniques, it aims to reduce a dilated pouch and/or gastrojejunal anastomosis to restore restriction, improve satiety, and optimize metabolic outcomes.
Gastric Bypass Revision in Los Angeles: When Weight Loss Stalls, a Second Chance Can Begin
For many patients, gastric bypass surgery is life-changing-improving weight,…

Top 10 Breakthrough Ingredients in the Cosmetics Industry in May 2025
With cosmetic products [https://www.topfeelbeauty.com/products/] becoming more and more segmented and supervision becoming more and more refined, the market has put forward higher requirements for the compliance, efficacy and safety of cosmetic ingredients. The dynamic changes in filing data directly reflect the industry trend. This article is based on the data of the top 10 ingredients with the highest filing growth rate in April 2025, which may provide the industry with…

Aluminum Takes the Lead in Curtain Wall Revolution
With the accelerating pace of urbanization, architectural curtain walls are more than just a building's exterior; they've become a concentrated expression of urban culture, technology, and environmental protection. As a core material in the curtain wall industry, aluminum, with its advantages of light weight, high strength, weather resistance, and excellent plasticity, is leading the curtain wall industry towards personalized, intelligent, and sustainable designs.
Lightweight and durable: the core strengths of aluminum…

Breakthrough in Volumetric 3D Printing: Hydrogel-Infused Composites Now Possible
In a significant advancement for volumetric 3D printing [https://www.skyone-pre.com/products/] (VAM), researchers from Switzerland's Ecole Polytechnique Federale de Lausanne (EPFL) and Sweden's Uppsala University have developed a pioneering volumetric additive manufacturing technique that enables post-printing hydrogel infusion-unlocking the creation of functional magnetic and conductive composites. This innovation addresses a longstanding limitation of traditional VAM, which has historically struggled to integrate soft, functional materials into printed structures, restricting its use in fields…
More Releases for Eosinophilic
Eosinophilic Esophagitis Market to Surge to USD 4.1 Billion by 2034
Eosinophilic Esophagitis (EoE) is a chronic, immune-mediated inflammatory condition of the esophagus, characterized by eosinophil infiltration, difficulty swallowing, food impaction, and chest pain. Once considered rare, EoE has now emerged as one of the most common causes of dysphagia in children and adults, particularly in developed nations. Strong associations with allergic conditions such as asthma, eczema, and food allergies underscore its complex immunological nature.
Download Full PDF Sample Copy of Market…
Eosinophilic Asthma Treatment Market Outlook and Future Projections for 2030
The eosinophilic asthma treatment market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines the…
Severe Eosinophilic Asthma Treatment Market Outlook and Future Projections for 2 …
The severe eosinophilic asthma treatment market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines…
Eosinophilic Esophagitis Pipeline, Clinical Trials Assessment, Emerging Therapie …
DelveInsight's, "Eosinophilic Esophagitis Pipeline Insight" report provides comprehensive insights about 25+ companies and 30+ pipeline drugs in Eosinophilic Esophagitis pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore our latest breakthroughs in Eosinophilic Esophagitis research. Learn more about…
Severe Eosinophilic Asthma Treatment Market Outlook and Future Projections for 2 …
The severe eosinophilic asthma treatment market represents a dynamic and continually evolving landscape, shaped by changing consumer demands and technological advancements. In this comprehensive report, we provide an in-depth exploration of the market, designed for a wide range of stakeholders including manufacturers, suppliers, distributors, and investors. Our goal is to equip industry participants with essential insights that enable informed decision-making in an ever-changing market environment. This analysis not only examines…
Eosinophilic Granulomatosis Treatment Market Size and Overview Upto 2028|
The report comes out as an intelligent and thorough assessment tool as well as a great resource that will help you to secure a position of strength in the global Eosinophilic Granulomatosis Treatment market. It includes Porter's Five Forces and PESTLE analysis to equip your business with critical information and comparative data about the Global Eosinophilic Granulomatosis Treatment Market. We have provided a deep analysis of the vendor landscape to give…
