Press release
Creative Diagnostics Launches Animal-Derived DNA Residue Assay Kits (qPCR) for Research Applications
Creative Diagnostics announces the launch of its new Animal-Derived DNA Residue Assay Kits (qPCR) for researchers.Creative Diagnostics, a reagent supplier and developer focused on biologics quality control, announces the launch of its new Animal-Derived DNA Residue Assay Kits [https://qbd.creative-diagnostics.com/products/animal-derived-dna-residue-assay-kits-qpcr-2791.html] (qPCR) for researchers to rapidly detect and quantify residual animal DNA in various samples. These kits offer exceptional specificity and sensitivity, ensuring product safety and compliance in the biopharmaceutical and biotech industries.
Creative Diagnostics has developed a range of host cell DNA assay kits for use in specific cell line expression systems based on rapid qPCR technology, which can be used for highly sensitive, highly accurate detection and quantification of host cell DNA contamination. These residual DNA molecules present in the human body along with biological products can lead to increased risk of carcinogenesis, infection and immunomodulation. The World Health Organisation (WHO) and the European Union (EU) allow residual DNA levels of up to 10 ng/dose and the US Food and Drug Administration (FDA) allows residual DNA levels of up to 100 pg/dose. To meet these requirements, methods with high sensitivity and accuracy are needed to detect and quantify low levels of DNA.
The newly released Animal-Derived DNA Residue Assay Kits utilize advanced qPCR technology to provide rapid and reliable results. By targeting specific animal DNA sequences, these kits enable precise detection and quantification of even trace amounts of residual animal material, minimizing the safety risks of animal-derived components and safeguarding public health. These innovative Animal-Derived DNA Residue Assay Kits, such as the Porcine DNA Residue Assay Kit and the Bovine DNA Residue Assay Kit, are essential tools for ensuring the integrity of biological products such as therapeutic proteins, vaccines, and antibodies.
For example, the Bovine DNA Residue Assay Kit (Cat. No. DDNA-017) is a PCR-based fluorescence probe assay for the quantitative detection of bovine DNA residues in biological samples. Offering high sensitivity and specificity, the kit can rapidly detect bovine DNA at the femtogram level. It includes a bovine DNA quantitative reference standard and is paired with the Creative Diagnostics MagIso Trademark Animal-derived Biomaterials Residual DNA Extraction Kits (Magnetic Beads) for optimal sample preparation. This assay is ideal for the accurate measurement of bovine DNA residues in various biomaterials, such as membranes, patches, and decellularized matrices.
The ResDetFast Trademark Human DNA Residue Assay Kit (Cat. No. DDNAF-018) is a rapid and sensitive qPCR assay for the quantitative detection of residual human DNA in biological products. It employs PCR primers and probes targeting conserved human genome sequences and includes a ready-to-use human DNA reference standard. When combined with the Creative Diagnostics Nucleic Acid Extraction or Purification Kit, the assay can detect femtogram levels of human DNA in just 1.5 hours. This kit is specifically designed for the quantitative detection of human host cell DNA in intermediate, semi-finished, and finished biological products.
For more information on custom Animal-Derived DNA Residue Assay Kits (qPCR) or project-specific Host Cell DNA Assay Kit development services, please visit https://qbd.creative-diagnostics.com/products/animal-derived-dna-residue-assay-kits-qpcr-2791.html.
About Creative Diagnostics
Creative Diagnostics is a global leader in the development and manufacturing of innovative tools and reagents for bioprocess impurity analysis. The company offers a comprehensive portfolio of solutions to support researchers in the quality control of biologics and provides biopharmaceutical quality, purity and safety assays, analytical methods and applications for the biotechnology and biopharmaceutical industries.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=creative-diagnostics-launches-animalderived-dna-residue-assay-kits-qpcr-for-research-applications]
State: New York
Country: United States
Website: https://qbd.creative-diagnostics.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Creative Diagnostics Launches Animal-Derived DNA Residue Assay Kits (qPCR) for Research Applications here
News-ID: 3712777 • Views: …
More Releases from ABNewswire

Dr. Zenovia Bryant-Bright Announces Upcoming Memoir: A Black Love Story of Loss, …
Image: https://www.abnewswire.com/upload/2026/01/45ff470dbcae7669354b6505319ce953.jpg
United States - Dr. Zenovia Bryant-Bright announces the release of her highly anticipated memoir, A Black Love Story of Loss, and Life After the Military , arriving January 26, 2026. This deeply personal and courageous book is more than a collection of memories, it is a testimony of survival, strength, love, and truth.
Too often, the stories of Black women who have served in the military are erased, dismissed, or…
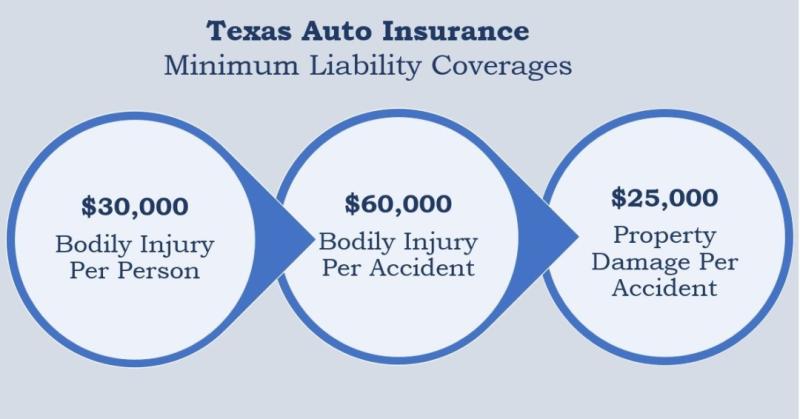
GetInsuredTexas.com Launches as the Essential Guide to Texas Insurance Policies, …
GetInsuredTexas.com, a new website from Baxter Insurance Agency, Inc., is now live, offering a one-stop shop for Texans to navigate the complexities of the state's insurance market, from understanding policies to comparing companies.
HOUSTON, TX - Jan 11, 2026 - Baxter Insurance Agency, Inc., a trusted name in the Texas insurance industry for over 40 years, is proud to announce the launch of GetInsuredTexas.com. This new platform is designed to be…

AI and the Future of the Translation Profession - Translation Conference to Deli …
Want to work smarter with AI - not just "go with the flow"? At the conference we'll share tools, methods, and practical workflows from the field - not just theory. Upgrade your professional toolkit.
The 2026 ITA hybrid translation conference will focus on one of the most urgent needs of today's language professionals: practical, applicable knowledge for working effectively - and responsibly - with artificial intelligence in real-world translation environments.
While public…

Caliente Brands Brings Bold Heat to Premium Beef Jerky Market with Authentic Spi …
Caliente Brands, LLC launches CalienteJerky.com, offering hickory-smoked beef jerky crafted exclusively from 100% USDA Approved Beef with uniquely spicy flavor profiles. The veteran-owned company targets consumers seeking bold, adventurous snack options with flavors ranging from Spicy Peppered to innovative Spicy Birria, delivering what the brand promises: where smoky and spicy meet delicious.
Caliente Brands, LLC has officially entered the premium beef jerky market with a clear mission: to deliver authentic spicy…
More Releases for DNA
High-Quality Plasmid DNA Fuels Growth in Global DNA Plasmid Manufacturing Market
🌍 Market Overview
The DNA Plasmid Manufacturing Market is experiencing robust growth as advancements in cell & gene therapy, DNA vaccines, and genetic engineering continue to expand globally. Plasmid DNA plays a critical role as a raw material in the development of advanced therapies, fueling demand across biopharmaceutical research and production.
Key factors driving the market include:
Increasing adoption of gene and cell therapies
Rising prevalence of chronic and rare genetic disorders
Expansion of DNA-based…
DNA Synthesis Market Increasing Demand for Synthetic Genes and DNA Sequences
As demonstrated by Precision Business Insights (PBI), the latest report, the global DNA synthesis market was valued at USD 3,702.0 million in 2023 and is expected to reach USD 10,289.5 million by 2029, growing at a CAGR of 18.6% during the forecast period 2024-2030. The key drivers for the growth of the global DNA synthesis market include increasing demand for synthetic genes and DNA sequences, growing applications in the agriculture…
Wealth DNA Code Review Legit Price? (Wealth Manifestation DNA Code Audio Frequen …
Wealth DNA Code Wealth DNA Code is a digital program with seven minutes of soundtracks that manifest and listen to daily to activate the "Wealth DNA," which is part of your DNA to help you attract wealth by making money a part of your mentality and making your dreams to come true.
https://bit.ly/Visit-The-Official-Website-Here-To-Order-Wealth-DNA-Code
Making money, creating assets as well as increasing wealth are the primary objectives that every human being has to…
DNA Paternity Testing Market Size [2022-2029] -DNA Diagnostics Center, EasyDNA, …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global DNA Paternity Testing. On the basis of historic growth analysis and current scenario of DNA Paternity Testing place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
DNA Paternity Testing Market Trends 2020 | Growth by Top Companies: DNA Diagnost …
The report begins with the overview of the DNA Paternity Testing Market and offers throughout development. It presents a comprehensive analysis of all the regional and major player segments that gives closer insights upon present market conditions and future market opportunities along with drivers, trending segments, consumer behaviour, pricing factors and market performance and estimation. The forecast market information, SWOT analysis, DNA Paternity Testing market scenario, and feasibility study are…
DNA Paternity Testing Market Rapidly Growing in Healthcare, Competitor Analysis …
The exclusive research report on the Global DNA Paternity Testing Market 2020 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global DNA Paternity Testing Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Overview of Global DNA Paternity Testing Market:
This report studies the Global DNA Paternity Testing…
