Press release
GMP Cell Therapy Consumables Market: Ensuring Quality and Compliance in Advanced Therapies
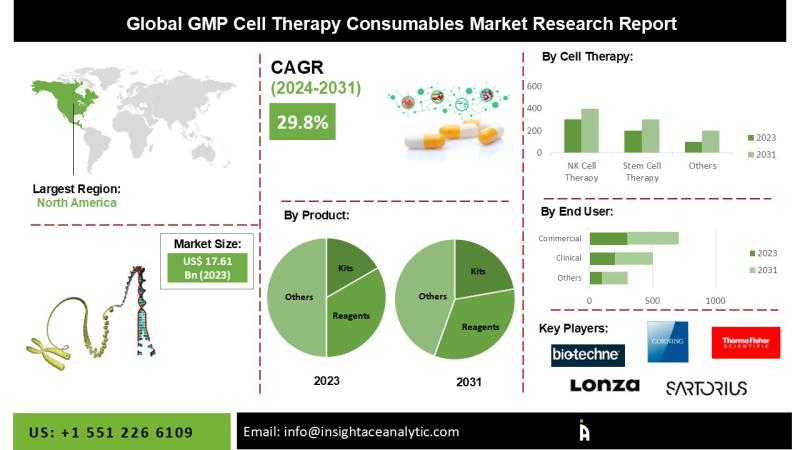
"GMP Cell Therapy Consumables Market" in terms of revenue was estimated to be worth $17.61 Bn in 2023 and is poised to reach $137.97 Bn by 2031, growing at a CAGR of 29.8% from 2024 to 2031 according to a new report by InsightAce Analytic.Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/1929
Market Analysis:
GMP (Good Manufacturing Practice) cell therapy consumables are materials used in the production and processing of cell-based therapies that meet stringent regulatory quality and safety requirements. The market for GMP cell therapy consumables is driven by a number of reasons, including the expansion and development of the cell therapy sector, regulatory requirements, and technological advances.
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global GMP Cell Therapy Consumables Market are:
• Increasing Demand for Cell Therapies
• Investment in Infrastructure and Manufacturing Capacity
• Advancements in Cell Therapy Technologies:
The following are the primary obstacles to the GMP Cell Therapy Consumables Market's expansion:
• High Initial Cost of GMP Cell Therapy Compliance
• Supply Chain Disruptions
• Limited Standardization and Collusion
Future expansion opportunities for the global GMP Cell Therapy Consumables Market include:
• Expansion of Indications and Therapeutic Applications across the Healthcare Industry
• Collaborations and Partnerships
• Technological Innovations
Key Industry Insights & Findings from the Report:
• Factors driving this tremendous growth include an expanding range of cell therapy applications, a global increase in cell therapy studies, regulatory endorsements, technological advancements in therapeutic platforms, and strategic expansions in cell therapy manufacturing.
• The rising prevalence of chronic diseases is a major factor driving demand for GMP cell therapy consumables.
• North America dominated the market and accounted for a revenue share of global revenue in 2023.
• One of the significant concerns restraining industry growth are the need for a large amount of investment for infrastructure, quality control measures, and staff training.
List of Prominent Players in the GMP Cell Therapy Consumables Market:
• Sartorius AG
• Thermo Fisher Scientific Inc
• Miltenyi Biotec BV & Co KG
• Bio-Techne Corp
• Corning Inc
• FUJIFILM Irvine Scientific Inc
• Lonza Group AG
• BPS Bioscience Inc
• Merck KGaA
• Global Life Sciences Solutions USA LLC
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1929
Recent Developments:
• In June 2023, STEMCELL Technologies entered into an agreement with PBS Biotech, under which the former will offer 3D cell culture media to enable the robust scaling-up of human pluripotent stem cell cultures in the latter's single-use bioreactor.
• In March 2023, GeminiBio, a leading manufacturer of cell culture materials and custom cGMP bioprocess liquids, announced the inauguration of a new, cutting-edge cGMP bioprocess liquid production facility in California, USA. According to the company's website, the new facility will offer customers custom-made cGMP cell culture media, buffers, and other process liquids in batch sizes of up to 10,000 liters.
• In January 2021, Sartorius formed a strategic partnership with RoosterBio Inc., a prominent provider of human mesenchymal stem/stromal cells (hMSC). The collaboration accelerated the growth of hMSC production for regenerative medicine.
GMP Cell Therapy Consumables Market Dynamics:
Market Drivers: Increasing Demand for Cell Therapies
The rising prevalence of chronic diseases is a major factor driving demand for GMP cell therapy consumables. Current research activities focusing on cell therapy as a possible treatment for chronic illnesses highlight the growing need for high-quality consumables to ensure the efficacy of clinical trials and therapy administration. Moreover, the market's growth is also being aided by new advancements in drug discovery, which are fueled by cutting-edge technologies in molecular biology, genetics, and high-throughput screening. The approval of multiple new pharmacological entities indicates a good trend in drug discovery activities, which will expand the market for GMP cell therapy consumables.
Challenges: High Initial Cost of GMP Cell Therapy Compliance
Achieving and sustaining GMP compliance necessitates large investments in infrastructure, quality control procedures, and employee training. The severe regulatory requirements increase the cost of manufacturing GMP-compliant consumables, potentially leading to increased prices for these products. Smaller businesses and academic organizations with insufficient financial means may find it difficult to cope. Additionally, the production processes for GMP cell therapy consumables can involve many phases and necessitate specialized equipment and knowledge. Manufacturers may face challenges in increasing production efficiency while maintaining product quality and regulatory compliance.
North America Is Expected To Grow With The Highest CAGR During The Forecast Period
The North America GMP Cell Therapy Consumables Market is likely to register a significant revenue share and develop at a rapid CAGR in the near future. This is due to a growth in medication development, R&D activity, and strategic collaborations among market players. Furthermore, rising rates of cancer, infectious diseases, autoimmune disorders, and neurological disorders have increased demand for personalized treatment and regenerative medicine, driving the expansion of the GMP cell therapy consumables market. The United States has a sizable share of the North American GMP cell therapy consumables industry. Rising government health expenditure, increased pharmaceutical industry development, and rising demand for innovative pharmaceuticals as a result of the incidence of numerous infectious diseases are all driving market expansion.
Segmentation of GMP Cell Therapy Consumables Market-
By Product-
• Kits
• Reagents/Molecular Biology Reagents
• Growth Factors/Cytokines and Interleukins
• Others
By Cell Therapy Type-
• NK Cell Therapy
• Stem Cell Therapy
• T-Cell Therapy
• Others
By Process-
• Cell Collection and Characterization/Sorting and Separation
• Cell Culture and Expansion/Preparation
• Cryopreservation
• Cell Processing and Formulation
• Cell Isolation and Activation
• Cell Distribution/Handling
• Process Monitoring and Control/Administration/Quality Assurance
• Others
By End-User
• Clinical
• Commercial
• Research
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1929
info@insightaceanalytic.com
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
Twitter: https://twitter.com/Insightace
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Cell Therapy Consumables Market: Ensuring Quality and Compliance in Advanced Therapies here
News-ID: 3666888 • Views: …
More Releases from InsightAce Analytic Pvt. Ltd
Sustainable Packaging Market Strategic Industry Overview and Forecast 2026 to 20 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Sustainable Packaging Market Size, Share & Trends Analysis Report By Material (Paper & Paperboard, Plastic, Metal, Glass), Process (Recycled, Reusable, Degradable), Function (Active, Molded Pulp, Alternate Fiber), Application (Food & Beverage, Healthcare, Others) & Layer (primary, secondary, and tertiary)- Market Outlook And Industry Analysis 2034"
The global Sustainable Packaging Market is estimated to reach over USD…
SCADA Market Insights Highlighting Technological Advancements in Wireless Sensor …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global SCADA Market Size, Share & Trends Analysis Report By Offering (Hardware, Software, Services), Component (Programmable Logic Controller, Remote Terminal Unit, Human-Machine Interface), End User (Process Industries, Discrete Manufacturing, Utilities), Region, Market Outlook And Industry Analysis 2034"
The global SCADA market is estimated to reach over USD 25.0 billion by the year 2034, exhibiting a CAGR of…
Biocomputer Based on Organoids Market Poised for 16.3% CAGR Driven by Brain Orga …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Biocomputer Based on Organoids Market Size, Share & Trends Analysis Report By Organoid Type (Brain Organoids, Other Organoids), Application (Biological Computing, Neuroscience Research, Drug Discovery and Testing, Personalized Medicine, Regenerative Medicine), Technology (Microfluidic Systems Microelectrode Arrays (MEAs), Brain-Machine Interfaces, CRISPR and Gene Editing), End-User (Academic and Research Institutes, Biotechnology and Pharmaceutical Companies, Technology Companies, Contract…
Postbiotic Supplements Market Drivers Include Functional Nutrition and Bioactive …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Postbiotic Supplements Market - (By Type (Bacteria, Yeast), By Form (Soft gels, Capsules/Tablets, Powder/ Granules, Others), By Application (Personal Care and Cosmetics, Food and Beverages, Animal Feed, Pharmaceuticals, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Postbiotic Supplements Market is valued at USD 12.8…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…