Press release
Retinitis Pigmentosa Pipeline Insights, Treatment Drugs, Clinical Trials, and Companies 2024
DelveInsight's, "Retinitis Pigmentosa Pipeline Insight 2024" report provides comprehensive insights about 45+ companies and 45+ pipeline drugs in Retinitis Pigmentosa pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our comprehensive Retinitis Pigmentosa Pipeline Report to stay informed about the latest advancements. Download copy now @ Retinitis Pigmentosa Pipeline Outlook [https://www.delveinsight.com/sample-request/retinitis-pigmentosa-retinitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Retinitis Pigmentosa Pipeline Report
* In September 2024:- Janssen Research & Development LLC- Phase 3 Randomized, Controlled Study of AAV5-hRKp.RPGR for the Treatment of X-linked Retinitis Pigmentosa Associated With Variants in the RPGR Gene. A clinical trial of AAV5-RPGR vector for participants with X-linked retinitis pigmentosa (XLRP)
* In September 2024:- AAVantgarde Bio Srl- A Phase 1/2 Multicenter, Open-label, Dose Escalation, Safety and Efficacy Study of Subretinal Administration of Dual AAV8.MYO7A, AAVB-081 in Subjects With Usher Syndrome Type IB (USH1B) Retinitis Pigmentosa. The purpose of the 081-101 study is to evaluate the safety and tolerability of a single subretinal injection of AAVB-081 in USH1B patients with retinitis pigmentosa due to a mutation in the MYO7A gene. The study will also assess the initial efficacy following AAVB-081 administration.
* DelveInsight's Retinitis Pigmentosa pipeline report depicts a robust space with 45+ active players working to develop 45+ pipeline therapies for Retinitis Pigmentosa treatment.
* The leading Retinitis Pigmentosa Companies such as Biogen, Neurotech, Ionis Pharmaceuticals, Novartis Pharmaceuticals, Nacuity Pharmaceuticals, ReNeuron, ID Pharma, Allegro Ophthalmics, SparingVision, Editas Medicine, OiDE OptoEye , and others.
* Promising Retinitis Pigmentosa Therapies such as EA-2353, BS01, CPK850, hRPC, SPVN06, RST-001 , and others.
Dive into our Retinitis Pigmentosa Pipeline Report to uncover promising therapies and breakthroughs. Gain insights that could shape the future of oncology @ Retinitis Pigmentosa Treatment Therapies [https://www.delveinsight.com/sample-request/retinitis-pigmentosa-retinitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Retinitis Pigmentosa Emerging Drugs Profile
* BIIB-112: Biogen
BIIB-112 (formerly called NSR-RPGR) consists of a standard AAV8 vector carrying a codon optimized RPGR gene. BIIB112 is in phase II clinical studies for X-linked retinitis pigmentosa treatment. In September 2018, NSR-RPGR was granted orphan drug designation for the treatment of inherited retinal dystrophies due to defects in the RPGR gene from the U.S. Food and Drug Administration.
* QR 1123 (formerly ISIS RHO 2.5Rx): Ionis Pharmaceuticals
QR 1123 (formerly ISIS RHO 2.5Rx) is an antisense oligonucleotide, designed to specifically target the mutant P23H messenger ribonucleic acid (mRNA) in order to reduce the expression of the P23H protein selectively, while preserving expression of the wild type (WT) rhodopsin (RHO) protein. The therapy is currently in Phase II clinical evaluation for the treatment of Retinitis Pigmentosa. In November 2019, ProQR Therapeutics announced that it received Orphan Drug designation (ODD) from the Food and Drug Administration (FDA) for QR-1123. QR-1123 was in-licensed from Ionis Pharmaceuticals in 2018. QR-1123 has also received Fast Track designation from the FDA.
Download the Retinitis Pigmentosa Pipeline Report to discover partnership opportunities and collaborate in driving impactful solutions forward @ Retinitis Pigmentosa Clinical Trials Assessment [https://www.delveinsight.com/sample-request/retinitis-pigmentosa-retinitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Retinitis Pigmentosa pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration
* Oral
* Parenteral
* Intravitreal
* Subretinal
* Topical
* Molecule Type
Retinitis Pigmentosa Products have been categorized under various Molecule types such as
* Monoclonal Antibody
* Peptides
* Polymer
* Small molecule
* Gene therapy
* Product Type
Dive into our detailed Retinitis Pigmentosa Pipeline Report to discover groundbreaking advancements shaping the future of cancer treatment @ Retinitis Pigmentosa Products, Companies, and Unmet Needs [https://www.delveinsight.com/sample-request/retinitis-pigmentosa-retinitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Retinitis Pigmentosa Pipeline Report
* Coverage- Global
* Retinitis Pigmentosa Companies- Biogen, Neurotech, Ionis Pharmaceuticals, Novartis Pharmaceuticals, Nacuity Pharmaceuticals, ReNeuron, ID Pharma, Allegro Ophthalmics, SparingVision, Editas Medicine, OiDE OptoEye, and others.
* Retinitis Pigmentosa Therapies- EA-2353, BS01, CPK850, hRPC, SPVN06, RST-001, and others.
* Retinitis Pigmentosa Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Retinitis Pigmentosa Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Gain valuable insights into emerging therapies and innovations with our Retinitis Pigmentosa Pipeline Report @ Retinitis Pigmentosa Market Drivers and Barriers, Future Perspectives and Analyst Views [https://www.delveinsight.com/sample-request/retinitis-pigmentosa-retinitis-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Retinitis Pigmentosa: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Retinitis Pigmentosa - DelveInsight's Analytical Perspective
* In-depth Commercial Assessment
* Retinitis Pigmentosa Collaboration Deals
* Late Stage Products (Phase III)
* Drug name: Company name
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* BIIB-112: Biogen
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* N-acetylcysteine-amide: Nacuity Pharmaceuticals
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* SPVN 06: SparingVision
* Drug profiles in the detailed report.....
* Inactive Products
* Retinitis Pigmentosa Key Companies
* Retinitis Pigmentosa Key Products
* Retinitis Pigmentosa- Unmet Needs
* Retinitis Pigmentosa- Market Drivers and Barriers
* Retinitis Pigmentosa- Future Perspectives and Conclusion
* Retinitis Pigmentosa Analyst Views
* Retinitis Pigmentosa Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=retinitis-pigmentosa-pipeline-insights-treatment-drugs-clinical-trials-and-companies-2024]
Phone: 9650213330
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: NV
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Retinitis Pigmentosa Pipeline Insights, Treatment Drugs, Clinical Trials, and Companies 2024 here
News-ID: 3666543 • Views: …
More Releases from ABNewswire

Yanik Guillemette: Securing Resilient Trade Amid CUSMA Challenges
As trade uncertainties rise, Quebec entrepreneur Yanik Guillemette emphasizes strategic diversification and robust protection for Canadian exports to ensure long-term stability.
Image: https://cdn.shopify.com/s/files/1/0719/9090/3064/files/Yanik_Guillemette_Quebec_Entrepreneur.jpg?v=1769600497
MONTREAL, Quebec - Feb 6, 2026 - The Canadian trade landscape is entering a period of significant recalibration. With the impending review of the Canada-United States-Mexico Agreement (CUSMA/USMCA) and the looming threat of cross-border tariffs, the nation's export-driven economy faces a critical test. Amidst this volatility, Yanik Guillemette [https://www.yanikguillemette.com/],…
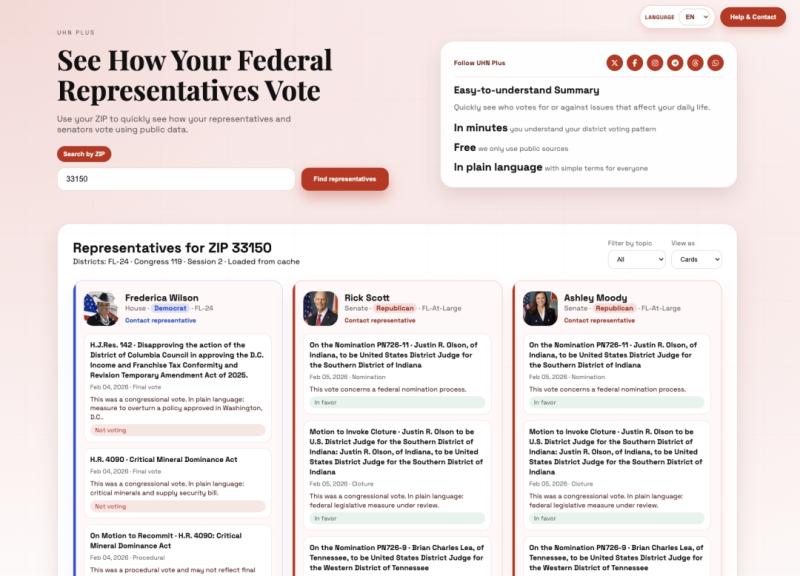
UHN Plus Launches 'How Do They Vote?' / '?Como votan?' - Free Bilingual Tool To …
UHN Plus launched "How Do They Vote?" (English) and "?Como votan?" (Spanish), a free bilingual platform to track congressional votes. The tool allows users to enter their ZIP code to see their representatives and their voting records on key issues. The platform aims to provide accessible, non-partisan information to empower voters ahead of the 2026 midterm elections.
UHN Plus, a leading digital media platform delivering real-time news and civic tools to…

Fertily.org launches an email-based fertility guidance service to help families …
Fertily.org has launched an email-based fertility guidance service that provides personalized, human support to individuals and couples navigating fertility treatment decisions. Through direct email correspondence with an experienced fertility coordinator, patients receive clear explanations of treatment options, cost guidance, clinic recommendations, and ongoing support.
Saint-Sauveur, Canada - February 6, 2026 - Fertily.org has officially launched as a personalized, email-correspondence fertility support service designed to help individuals and couples navigate the complexity…

When the Cold Hits -40 degrees C, Reliability Is About Who Shows Up: Winnipeg To …
After a December blizzard triggered over 2,700 roadside calls in a single weekend, Winnipeg Towing is reinforcing its 24/7 rapid-response fleet to meet surging winter demand across Manitoba. With extreme cold warnings and tow risks from parking bans on the rise, the company provides specialized flatbed towing, battery boost, car unlocking, and heavy-duty recovery across all Winnipeg neighbourhoods. Transparent upfront pricing and insurance-approved accident towing come standard on every call.
WINNIPEG,…
More Releases for Retinitis
Shaping the Retinitis Pigmentosa Market in 2025: Innovative Gene Therapy Develop …
How Big Is the Retinitis Pigmentosa Market Expected to Be, and What Will Its Growth Rate Be?
In recent times, a significant expansion has been witnessed in the market size of retinitis pigmentosa. The market is set to rise from $11.32 billion in 2024 to $12.22 billion in 2025, delivering a compound annual growth rate (CAGR) of 8.0%. The proliferation in the preceding period is due to progress in genetic…
Shaping the Retinitis Pigmentosa Market in 2025: Innovative Gene Therapy Develop …
How Big Is the Retinitis Pigmentosa Market Expected to Be, and What Will Its Growth Rate Be?
In recent times, a significant expansion has been witnessed in the market size of retinitis pigmentosa. The market is set to rise from $11.32 billion in 2024 to $12.22 billion in 2025, delivering a compound annual growth rate (CAGR) of 8.0%. The proliferation in the preceding period is due to progress in genetic…
Retinitis Pigmentosa Market Growth and Treatment Advancements
The retinitis pigmentosa (RP) market has garnered considerable attention in recent years due to its profound impact on individuals' lives. As one of the leading causes of hereditary blindness, RP is a genetic eye disorder that progressively leads to vision loss. The market for retinitis pigmentosa treatments was valued at USD 14.02 billion in 2023, and with significant advancements on the horizon, it is expected to reach USD 21.40 billion…
Retinitis Pigmentosa Market Research and Market Share Insights
The Business Research Company recently released a comprehensive report on the Global Retinitis Pigmentosa Market Size and Trends Analysis with Forecast 2024-2033. This latest market research report offers a wealth of valuable insights and data, including global market size, regional shares, and competitor market share. Additionally, it covers current trends, future opportunities, and essential data for success in the industry.
Ready to Dive into Something Exciting? Get Your Free Exclusive Sample…
Cytomegalovirus Retinitis Market Forecast 2031: Growth and Opportunities
The "Cytomegalovirus Retinitis Market" is a dynamic and rapidly evolving sector, with significant advancements and growth anticipated by 2031. Comprehensive market research reveals a detailed analysis of market size, share, and trends, providing valuable insights into its expansion. This report delves into segmentation and definition, offering a clear understanding of market components and drivers. Employing SWOT and PESTEL analyses, the study evaluates the market's strengths, weaknesses, opportunities, and threats, alongside…
Retinitis Pigmentosa (Retinitis) Market Analysis and Future Prospects for 2030
The world of the retinitis pigmentosa (retinitis) market is a complex and ever-evolving landscape, shaped by consumer demands and technological advancements. In this report, we delve into the depths of this market to provide a profound and comprehensive analysis, catering to a diverse audience that includes manufacturers, suppliers, distributors, and investors. Our primary goal is to empower industry stakeholders with invaluable insights to make informed decisions in a rapidly changing…
