Press release
e-Clinical Solutions Market: An In-Depth Analysis
Introduction:The e-Clinical solutions market is witnessing rapid growth as the healthcare industry increasingly turns to digital tools to enhance clinical trials and streamline data management. e-Clinical solutions integrate electronic systems and software for clinical trials, facilitating better data collection, management, and analysis. This market is a vital component of the modern clinical research landscape, allowing companies to conduct trials more efficiently and at a lower cost.
In this guest post, we will examine the e-Clinical solutions market in detail, covering aspects such as market size, market share, trends, growth, demand, and the factors driving this growth. As the global healthcare sector embraces digital transformation, understanding the dynamics of the e-Clinical solutions market becomes crucial for industry stakeholders.
Market Size:
The global e-clinical solutions market is fragmented in nature, as it consists of many global players such as Oracle, IQVIA Inc., Dassault Systemes, and Clario among others. The presence of these companies produces competitive prices for systems services and software across the region. Due to the presence of these players at regional and international levels, suppliers and manufacturers offer products with different specifications and characteristics in all budgets. The growing adoption of electronic data capture (EDC) systems and cloud-based is driving the market growth. Additionally, the growing focus on patient-centric clinical trials is expected to drive market growth.
https://www.databridgemarketresearch.com/reports/global-eclinical-solutions-market
Some of the major market players operating in the global e-clinical solutions market are Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, and RealTime Software Solutions LLC among others.
Market Share:
The e-Clinical solutions market is highly competitive, with several key players dominating the industry. Some of the major companies include Medidata Solutions (a Dassault Systèmes company), Oracle Health Sciences, Parexel International, CRF Health, and Veeva Systems. These companies hold a substantial share of the market due to their comprehensive product offerings, strong industry relationships, and continuous innovation.
Smaller companies and startups are also entering the market, focusing on niche areas such as patient engagement, real-world evidence, and advanced analytics. These new entrants are contributing to the market's dynamism by offering specialized solutions that cater to specific needs within clinical trials. As a result, the market is characterized by a mix of established players and emerging innovators, each contributing to the overall growth and diversity of the industry.
Market Trends:
Several key trends are shaping the e-Clinical solutions market. One of the most notable trends is the increasing adoption of cloud-based solutions. Cloud platforms offer flexibility, scalability, and accessibility, allowing researchers to access data and collaborate in real-time from different locations. This trend has gained momentum as clinical trials become more decentralized, with participants and researchers often dispersed across various geographic regions.
Another significant trend is the integration of artificial intelligence (AI) and machine learning (ML) into e-Clinical solutions. AI and ML can enhance data analysis, identify patterns, and predict outcomes, thereby improving the efficiency and accuracy of clinical trials. These technologies are particularly useful in managing large datasets, reducing the time required for data processing, and identifying potential issues early in the trial process.
The rise of patient-centric trials is also influencing the e-Clinical solutions market. There is a growing emphasis on improving patient engagement and experience during clinical trials. e-Clinical solutions now often include tools for remote monitoring, telemedicine, and electronic patient-reported outcomes (ePRO), allowing participants to provide data from the comfort of their homes. This trend not only improves patient compliance but also enables more diverse and representative trial populations.
Moreover, regulatory compliance is a critical trend driving the development of e-Clinical solutions. Regulatory agencies such as the FDA and EMA have increasingly stringent requirements for data integrity, transparency, and patient safety. As a result, e-Clinical solutions are evolving to ensure that they meet these regulatory standards, offering features like audit trails, electronic signatures, and real-time reporting to ensure compliance throughout the trial process.
Market Growth:
The e-Clinical solutions market is poised for robust growth in the coming years, driven by several factors. The increasing number of clinical trials, particularly in areas such as oncology, rare diseases, and personalized medicine, is one of the primary drivers of market growth. As these trials become more complex and require more sophisticated data management, the demand for e-Clinical solutions will continue to rise.
Another factor contributing to market growth is the shift towards decentralized and virtual trials. The COVID-19 pandemic has accelerated the adoption of decentralized trial models, which rely heavily on digital tools for data collection, monitoring, and analysis. e-Clinical solutions play a critical role in enabling these trials, providing the infrastructure needed to manage data from multiple sources and ensure that trials remain compliant with regulatory standards.
The growing focus on cost efficiency in clinical research is also driving the market's expansion. Pharmaceutical companies are under pressure to reduce the time and cost associated with bringing new drugs to market. e-Clinical solutions offer a way to streamline trial processes, reduce data entry errors, and improve overall trial efficiency, leading to faster and more cost-effective trials.
Furthermore, the increasing adoption of real-world evidence (RWE) in clinical research is fueling demand for e-Clinical solutions. RWE involves collecting and analyzing data from real-world settings, such as electronic health records (EHRs) and wearable devices, to inform clinical trial design and decision-making. e-Clinical solutions provide the tools needed to integrate and analyze RWE data, making them an essential component of modern clinical research.
Market Demand:
The demand for e-Clinical solutions is being driven by several key factors. One of the main drivers is the need for more efficient and accurate data management in clinical trials. As trials become more complex and involve larger datasets, traditional paper-based and manual data management methods are no longer sufficient. e-Clinical solutions provide the tools needed to collect, manage, and analyze data more effectively, reducing errors and improving trial outcomes.
Another factor driving demand is the increasing regulatory requirements for clinical trials. Regulatory agencies are placing greater emphasis on data integrity, transparency, and patient safety, making it essential for companies to adopt systems that ensure compliance. e-Clinical solutions offer the features needed to meet these requirements, such as electronic data capture (EDC), audit trails, and real-time reporting.
The growing trend towards patient-centric trials is also contributing to demand for e-Clinical solutions. Patients are increasingly expecting more convenient and personalized trial experiences, and e-Clinical tools enable remote monitoring, telemedicine, and electronic data submission. This trend is particularly important in light of the COVID-19 pandemic, which has made it difficult for patients to attend in-person visits and has increased the need for remote trial solutions.
The increasing use of data analytics in clinical research is another factor driving demand. Advanced analytics tools allow researchers to identify trends, predict outcomes, and make data-driven decisions. e-Clinical solutions that integrate AI and ML capabilities are becoming increasingly popular as companies look to leverage these technologies to improve trial efficiency and outcomes.
Factors Driving Growth:
Several factors are driving the growth of the e-Clinical solutions market. One of the most significant drivers is the increasing complexity of clinical trials. As trials involve more participants, sites, and data points, the need for sophisticated data management systems becomes critical. e-Clinical solutions offer the scalability and flexibility needed to manage these complexities, making them essential for modern clinical research.
Another key factor driving growth is the shift towards decentralized and virtual trials. The COVID-19 pandemic has highlighted the importance of digital tools in enabling remote trials, and this trend is expected to continue even after the pandemic subsides. e-Clinical solutions provide the infrastructure needed to support decentralized trials, allowing companies to conduct trials more efficiently and with greater flexibility.
The growing focus on patient-centric trials is also contributing to market growth. Patients are increasingly expecting more convenient and personalized trial experiences, and e-Clinical solutions enable companies to meet these expectations by offering remote monitoring, telemedicine, and electronic data submission options.
Moreover, the increasing adoption of real-world evidence in clinical research is fueling demand for e-Clinical solutions. RWE involves collecting and analyzing data from real-world settings, and e-Clinical solutions provide the tools needed to integrate and analyze this data, making them an essential component of modern clinical research.
Conclusion:
The e-Clinical solutions market is set for significant growth in the coming years, driven by the increasing complexity of clinical trials, the shift towards decentralized and virtual trials, and the growing focus on patient-centric trials. As the global healthcare industry continues to embrace digital transformation, the demand for e-Clinical solutions will only increase, making them a critical component of the future of clinical research.
Browse Trending Reports:
https://hgreenonenews.blogspot.com/2024/08/virtual-infrastructure-manager-market.html
https://sepnews02.blogspot.com/2024/09/breast-implants-accessories-market-size.html
https://sepnews02.blogspot.com/2024/09/radio-frequency-identification.html
https://sepnews02.blogspot.com/2024/09/natural-surfactant-market-size-share.html
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: corporatesales@databridgemarketresearch.com
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release e-Clinical Solutions Market: An In-Depth Analysis here
News-ID: 3644928 • Views: …
More Releases from Data Bridge Market Research

Scented Candle Market Shows Strong Growth Driven by Wellness and Home Décor Tr …
The global scented candle market is on track for significant expansion, increasing from an estimated USD 3.60 billion in 2024 to USD 6.00 billion by 2032, registering a strong CAGR of 6.60%. Rising consumer interest in home ambiance, wellness, and premium lifestyle products continues to drive market demand.
Get More Detail: https://www.databridgemarketresearch.com/reports/global-scented-candle-market
Market Growth Drivers
The scented candle market has evolved beyond being just a decorative item. Key growth factors include:
Home Fragrance &…
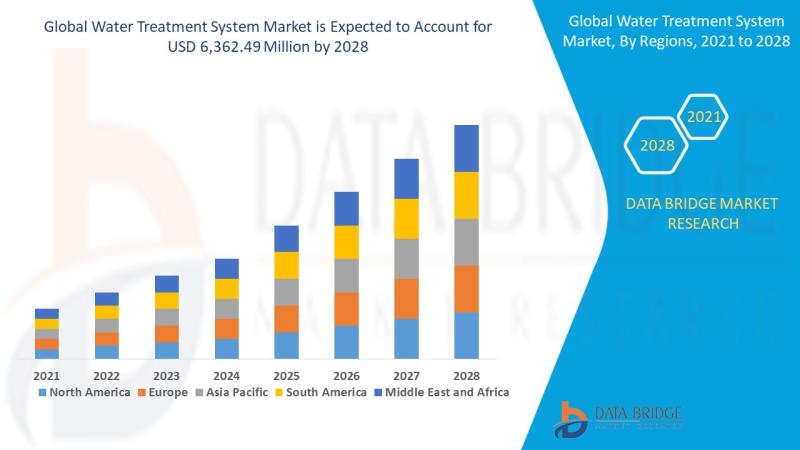
Water Treatment System Market: Sustaining the Future of Clean Water
Introduction
Understanding Water Treatment Systems
Water treatment systems are designed to purify and disinfect water for various uses-drinking, industrial processes, irrigation, and wastewater reuse. These systems eliminate contaminants such as bacteria, viruses, heavy metals, chemicals, and particulates, making water safe and sustainable for consumption and use.
Importance in Global Sustainability
Clean water is essential to life and industrial progress. With growing water demand and pollution, water treatment systems are now critical infrastructure across the…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…

Veterinary X-Ray Market Size, Analysis, Scope, Demand, Opportunities, Statistics
According to Data Bridge Market Research The global Veterinary X-Ray market size was valued at USD 915.19 million in 2024 and is projected to reach USD 1576.00 million by 2032, with a CAGR of 7.03 % during the forecast period of 2025 to 2032.
With increasing globalization and digital disruption, the Equine X-Ray Solutions Market is expanding across multiple industries, . Market research data indicates that businesses in the Companion Animal…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
