Press release
eClinical Solutions Market Set to Reach $23.32 Billion by 2031: Innovations and Opportunities in Clinical Research
As the healthcare and life sciences sectors continue to evolve, the demand for advanced data management solutions has never been greater. This growing need is propelling the eClinical solutions market into the spotlight, with projections indicating a significant rise in market value to $23.32 billion by 2031. This represents a robust compound annual growth rate (CAGR) of 13.9% from 2024 to 2031, according to the latest publication from Meticulous Research®. This anticipated growth is underpinned by several critical factors, including the massive volumes of data being generated within the healthcare industry, the increasing number of clinical trials, government and private sector initiatives supporting clinical research, and a surge in funding dedicated to medical and pharmaceutical research and development (R&D). Additionally, efforts to enhance IT infrastructure across major hospital networks and the growing demand for precision medicine are expected to create significant opportunities for players in the eClinical solutions market.Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5910
Market Dynamics: Key Drivers Fueling Growth
The eClinical solutions market is set on a path of robust expansion, driven by several key factors:
1. Data Proliferation in Healthcare and Life Sciences
The healthcare and life sciences industries are generating unprecedented amounts of data due to advancements in medical research, patient care, diagnostics, and digital health technologies. This data explosion is a double-edged sword, offering vast potential for insights and innovation while also presenting challenges in terms of data management, analysis, and security. The growing complexity and volume of data are driving the adoption of eClinical solutions, which are designed to handle large datasets efficiently and accurately. These solutions enable healthcare organizations to streamline their operations, improve patient outcomes, and accelerate research and development processes.
2. Rise in Clinical Trials
The global increase in the number of clinical trials is another significant factor contributing to the growth of the eClinical solutions market. As pharmaceutical companies, biopharmaceutical firms, and medical device manufacturers strive to develop new therapies and treatments, the demand for efficient and reliable clinical trial management systems has surged. eClinical solutions play a critical role in managing the various stages of clinical trials, from patient recruitment and data collection to analysis and reporting. These solutions ensure that clinical trials are conducted with high levels of accuracy, compliance, and efficiency, thereby reducing the time and cost associated with bringing new treatments to market.
3. Supportive Government Initiatives and Funding
Governments and private organizations worldwide are increasingly recognizing the importance of clinical research in advancing healthcare. This recognition has led to the implementation of various initiatives aimed at supporting clinical research and development. For example, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have introduced guidelines and frameworks to facilitate the approval of new drugs and medical devices. Additionally, increased funding for medical and pharmaceutical R&D is driving the adoption of eClinical solutions, as organizations seek to leverage advanced technologies to enhance their research capabilities.
4. Strengthening of IT Infrastructure
Large hospital networks and healthcare providers are investing heavily in strengthening their IT infrastructures to support the adoption of advanced data management systems. This trend is particularly evident in developed regions, where healthcare providers are increasingly adopting eClinical solutions to enhance patient care, improve operational efficiency, and ensure compliance with regulatory requirements. By integrating eClinical solutions into their IT infrastructures, healthcare organizations can streamline their data management processes, reduce errors, and improve the overall quality of care.
5. Growing Demand for Precision Medicine
Precision medicine, which tailors treatment plans based on individual patient characteristics such as genetics, lifestyle, and environment, is gaining significant traction in the healthcare industry. The increasing adoption of precision medicine is driving the demand for eClinical solutions that can support personalized data analysis and patient care management. These solutions enable healthcare providers to collect and analyze large volumes of patient data, identify patterns and trends, and develop targeted treatment plans that improve patient outcomes.
Key Players: Leading Innovators in the eClinical Solutions Market
The eClinical solutions market is characterized by a dynamic landscape of key players who are at the forefront of driving innovation and growth. These companies are instrumental in developing and implementing cutting-edge eClinical solutions that are transforming the way clinical research and healthcare data management are conducted. The major players in the eClinical solutions market include:
• Dassault Systems S.E. (France): A global leader in 3D design software, Dassault Systems has expanded its capabilities into the life sciences sector, offering comprehensive eClinical solutions that support clinical trial management, data analysis, and regulatory compliance.
• Fountayn (U.S.): Known for its innovative cloud-based solutions, Fountayn provides a range of eClinical tools that streamline clinical trial processes, enhance data management, and improve collaboration among research teams.
• Merative L.P. (U.S.): Formerly known as IBM Watson Health, Merative specializes in advanced analytics and AI-driven solutions that enable healthcare organizations to optimize their clinical research efforts and improve patient
outcomes.
• eClinical Solutions LLC (U.S.): A leading provider of cloud-based data management solutions, eClinical Solutions LLC offers a comprehensive suite of tools that facilitate efficient clinical trial management and data integration.
• Clario (U.S.): Clario provides a range of eClinical solutions that focus on improving data quality, reducing trial timelines, and ensuring regulatory compliance, making it a key player in the industry.
• eClinicalWorks, LLC (U.S.): With a focus on electronic health records (EHR) and practice management, eClinicalWorks offers integrated eClinical solutions that support clinical research and patient care.
• IQVIA Inc. (U.S.): A global leader in advanced analytics, technology solutions, and clinical research services, IQVIA plays a pivotal role in the eClinical solutions market, offering innovative tools that drive efficiency and accuracy in clinical trials.
• Parexel International (MA) Corporation (U.S.): Parexel is a leading provider of clinical research services, offering comprehensive eClinical solutions that support the entire clinical trial lifecycle, from study design to data analysis.
• MaxisIT Inc. (U.S.): MaxisIT specializes in providing integrated eClinical solutions that enable seamless data management, collaboration, and analysis, helping organizations accelerate their clinical research efforts.
• Signant Health (U.S.): Signant Health offers a range of eClinical solutions that focus on patient engagement, data capture, and trial management, ensuring the success and reliability of clinical trials.
• Castor (U.S.): Castor provides cloud-based eClinical solutions that streamline data collection and management, enabling researchers to conduct efficient and accurate clinical trials.
• Veeva Systems Inc. (U.S.): Veeva Systems is a leading provider of cloud-based software solutions for the life sciences industry, offering a range of eClinical tools that support clinical trial management, regulatory compliance, and data analysis.
• Oracle Corporation (U.S.): Oracle offers a comprehensive
suite of eClinical solutions that leverage advanced analytics, cloud computing, and AI to enhance clinical trial management and data integration.
These industry leaders are continuously innovating and expanding their product offerings to meet the evolving needs of the eClinical solutions market, ensuring that healthcare organizations have access to the most advanced tools and technologies available.
Customize Your Report : https://www.meticulousresearch.com/request-customization/cp_id=5910
Market Segmentation: A Detailed Analysis of Market Segments
The eClinical solutions market is segmented into various categories based on product type, clinical trial phase, end user, and geography. This segmentation provides a comprehensive understanding of the market's diverse landscape and highlights the areas poised for significant growth.
Product Segmentation: Diverse Solutions for Complex Needs
The eClinical solutions market is segmented by product type into several categories, each catering to different aspects of clinical research and data management. These product categories include:
1. Clinical Data Management Systems (CDMS): CDMS are essential for managing the vast amounts of data generated during clinical trials. These systems ensure that data is collected, stored, and analyzed accurately, maintaining compliance with regulatory standards and supporting the overall success of clinical trials.
2. Clinical Trial Management Systems (CTMS): The CTMS segment is projected to register the highest growth rate of 13.5% during the forecast period. The increasing number of clinical trials, coupled with the growing demand for organized and reliable clinical trial data, is driving the adoption of CTMS. These systems are crucial for ensuring the quality, accuracy, and integrity of clinical trial data, making them indispensable tools for pharmaceutical companies, CROs, and other stakeholders in the clinical research process.
3. Randomization & Trial Supply Management Systems: These systems are integral to the randomization process in clinical trials, ensuring that patients are assigned to treatment groups in a randomized manner. This process is critical for minimizing bias and ensuring the validity of trial results, making these systems a key component of the eClinical solutions market.
4. Electronic Data Capture (EDC): EDC systems are widely adopted for their ability to streamline data collection processes in clinical trials. These systems reduce errors, accelerate data analysis, and improve the overall efficiency of clinical research, making them a popular choice among pharmaceutical companies and CROs.
5. Electronic Clinical Outcome Assessments (eCOA) and Electronic Patient-reported Outcomes (ePRO): These systems enable the collection of patient-reported data, providing valuable insights into treatment efficacy and patient experiences. eCOA and ePRO systems are increasingly being adopted in clinical trials, as they offer a more patient-centric approach to data collection and analysis.
6. Clinical Analytics Platforms: Clinical analytics platforms offer advanced data analytics capabilities, allowing researchers to derive actionable insights from clinical trial data. These platforms are essential for making informed decisions about treatment efficacy, patient safety, and regulatory compliance.
7. Electronic Trial Master File (eTMF): eTMF systems are critical for maintaining and managing the documentation required for regulatory compliance in clinical trials. These systems ensure that all trial-related documents are securely stored and easily accessible, supporting the overall efficiency and transparency of clinical research.
8. Clinical Data Integration Platforms: These platforms facilitate the integration of data from various sources, enabling a comprehensive view of clinical trial outcomes. Clinical data integration platforms are essential for ensuring that all relevant data is available for analysis, helping researchers make informed decisions and improve trial outcomes.
9. Safety Solutions: Safety solutions are crucial for monitoring and managing adverse events during clinical trials. These systems ensure that patient safety is maintained throughout the trial process, while also supporting regulatory compliance and data integrity.
10. Other Product Types: This category includes various other eClinical solutions that support different aspects of clinical research and data management. These products may include specialized tools for specific trial phases, data types, or regulatory requirements.
Clinical Trial Phase Segmentation: Understanding Market Dynamics
The eClinical solutions market is also segmented based on the phase of clinical trials, with each phase presenting unique requirements and challenges. These phases include:
1. Phase I: Phase I trials are the initial stage of clinical testing, focusing on assessing the safety and dosage of new treatments. eClinical solutions are essential for managing and analyzing the data generated during this phase, ensuring that trials are conducted safely and efficiently.
2. Phase II: Phase II trials are designed to evaluate the efficacy of a treatment in a larger patient population. The demand for eClinical solutions in this phase is driven by the need for robust data management and analysis tools that can handle the increased complexity and volume of data.
3. Phase III: Phase III trials are the largest and most comprehensive phase of clinical testing, requiring advanced eClinical solutions to manage the vast amounts of data generated and ensure regulatory compliance. These solutions play a critical role in supporting the successful completion of Phase III trials, which are essential for obtaining regulatory approval for new treatments.
4. Phase IV: Phase IV trials, also known as post-marketing studies, are conducted after a treatment has been approved for use. These trials focus on monitoring the long-term safety and efficacy of treatments in a larger patient population. eClinical solutions are crucial for managing the ongoing data collection and analysis required in Phase IV trials, ensuring that treatments continue to meet regulatory standards and provide value to patients.
End User Segmentation: Identifying Key Market Participants
The eClinical solutions market serves a diverse range of end users, including pharmaceutical and biopharmaceutical companies, clinical research organizations (CROs), medical device manufacturers, and other stakeholders involved in clinical research. These end users include:
1. Pharmaceutical & Biopharmaceutical Companies: In 2024, pharmaceutical and biopharmaceutical companies are expected to account for the largest share of the eClinical solutions market. The significant number of clinical trials undertaken by these companies, coupled with their focus on accelerating drug research and development, drives the demand for eClinical solutions. For instance, the U.S. Food and Drug Administration's Center for Drug Evaluation and Research (CDER) approved 55 novel drugs in 2023, up from 37 in 2022, highlighting the growing focus on drug development and the need for efficient clinical trial management systems.
2. Clinical Research Organizations (CROs): CROs play a crucial role in conducting clinical trials on behalf of pharmaceutical and biopharmaceutical companies. The growing outsourcing of clinical trials to CROs is driving the adoption of eClinical solutions within this segment, as these organizations seek to enhance their data management capabilities and improve trial efficiency.
3. Medical Device Manufacturers: As the development of medical devices becomes increasingly complex, the demand for eClinical solutions to manage clinical trial data and ensure regulatory compliance is on the rise. Medical device manufacturers rely on eClinical solutions to streamline their clinical research processes, reduce errors, and accelerate time-to-market for new products.
4. Other End Users: This category includes academic research institutions, government agencies, and other organizations involved in clinical research. These end users are increasingly adopting eClinical solutions to support their research efforts, improve data management, and ensure compliance with regulatory standards.
Geographical Analysis: Regional Insights and Growth Opportunities
The eClinical solutions market is analyzed across major geographies, providing a comprehensive understanding of regional market dynamics and identifying key growth opportunities. The primary regions analyzed include:
1. North America: The North American eClinical solutions market, encompassing the U.S. and Canada, is a significant player in the global market. The region's well-established healthcare infrastructure, coupled with strong government support for clinical research, drives market growth. The presence of key industry players further strengthens the market position in this region, making North America a key hub for eClinical solutions innovation and adoption.
2. Europe: Europe is another key market for eClinical solutions, with countries such as Germany, France, the U.K., and Italy leading the charge. The region's focus on advancing healthcare research and development, along with stringent regulatory requirements, fuels the demand for eClinical solutions. European healthcare providers and research institutions are increasingly adopting these solutions to enhance their clinical research capabilities and improve patient outcomes.
3. Asia-Pacific: The Asia-Pacific region is projected to register the highest CAGR during the forecast period, driven by the rapidly growing pharmaceutical and biopharmaceutical industries in countries like India and China. The increasing number of clinical trials being conducted in the region, coupled with the growing preference for eClinical solutions over manual data management processes, presents lucrative opportunities for market players. Additionally, government initiatives to promote healthcare innovation and investment in digital health technologies are further driving the adoption of eClinical solutions in the Asia-Pacific region.
4. Latin America: The Latin American eClinical solutions market, including countries such as Brazil and Mexico, is witnessing growth driven by the expanding pharmaceutical industry and increasing clinical research activities in the region. Healthcare providers and research organizations in Latin America are increasingly adopting eClinical solutions to streamline their data management processes, improve trial efficiency, and ensure compliance with regulatory standards.
5. Middle East & Africa: The Middle East and Africa region is gradually adopting eClinical solutions as healthcare infrastructure improves and the demand for advanced data management solutions increases. While the market in this region is still in its early stages of development, there are significant growth opportunities as healthcare providers and research institutions invest in eClinical solutions to enhance their clinical research capabilities and improve patient care.
Challenges and Opportunities: Navigating the eClinical Solutions Market
Challenges Facing the eClinical Solutions Market
Despite the strong growth prospects for the eClinical solutions market, there are several challenges that could impact the market's trajectory. These challenges include:
1. Regulatory Compliance: The eClinical solutions market operates in a highly regulated environment, with strict guidelines and standards imposed by regulatory bodies such as the FDA, EMA, and other global health authorities. Ensuring that eClinical solutions meet these regulatory requirements can be complex and time-consuming, posing challenges for market players in terms of product development and compliance.
2. Data Security and Privacy: The increasing volume of healthcare data being generated and managed through eClinical solutions raises concerns about data security and privacy. Ensuring that patient data is protected from unauthorized access and breaches is a critical challenge for healthcare organizations and eClinical solutions providers. Failure to adequately address data security and privacy issues could result in regulatory penalties, loss of patient trust, and damage to the organization's reputation.
3. Integration with Legacy Systems: Many healthcare organizations continue to rely on legacy systems for data management, making it challenging to integrate new eClinical solutions into existing IT infrastructures. The lack of interoperability between different systems can hinder the seamless flow of data, resulting in inefficiencies and delays in clinical research processes.
4. High Implementation Costs: The cost of implementing eClinical solutions can be a barrier for smaller healthcare organizations and research institutions, particularly in developing regions where resources may be limited. High implementation costs can also deter organizations from adopting new technologies, slowing down the overall growth of the eClinical solutions market.
5. Complexity of Clinical Trials: As clinical trials become more complex, with increasing numbers of participants, multiple trial sites, and diverse data sources, managing the trial process becomes more challenging. Ensuring that eClinical solutions can handle the complexity of modern clinical trials is critical to the success of these solutions.
Opportunities in the eClinical Solutions Market
Despite the challenges, the eClinical solutions market presents several opportunities for growth and innovation. These opportunities include:
1. Advancements in Artificial Intelligence (AI) and Machine Learning (ML): The integration of AI and ML technologies into eClinical solutions offers significant potential for improving data analysis, predictive modeling, and decision-making in clinical trials. These technologies can help researchers identify trends and patterns in clinical trial data, optimize trial design, and improve patient outcomes.
2. Expansion into Emerging Markets: The growing healthcare infrastructure in emerging markets such as Asia-Pacific, Latin America, and the Middle East presents significant growth opportunities for eClinical solutions providers. As healthcare organizations in these regions invest in digital health technologies and modernize their IT infrastructures, the demand for eClinical solutions is expected to increase.
3. Collaboration with CROs and Pharma Companies: Collaboration between eClinical solutions providers and CROs or pharmaceutical companies can drive innovation and improve the efficiency of clinical trials. By working together, these organizations can develop new solutions that address the specific needs of clinical research, streamline trial processes, and reduce time-to-market for new treatments.
4. Personalized Medicine and Genomics: The growing focus on personalized medicine and genomics is driving demand for eClinical solutions that can support the collection and analysis of complex patient data. As researchers continue to explore the potential of personalized medicine, there is a growing need for advanced data management tools that can handle the complexity and scale of genomic data.
5. Regulatory Harmonization: Efforts to harmonize regulatory standards across different regions could simplify the development and implementation of eClinical solutions, reducing the burden of compliance for market players. Regulatory harmonization could also facilitate the global expansion of eClinical solutions providers, opening up new markets and opportunities for growth.
Read Full Report @ https://www.meticulousresearch.com/product/eclinical-solutions-market-5910
Read Our Report-
• Clinical Trials Market : https://www.meticulousresearch.com/product/clinical-trials-market-5934
• Patient Engagement Solutions Market : https://www.meticulousresearch.com/product/patient-engagement-solutions-market-5566
• U.S. Real-World Evidence (RWE) Solutions Market : https://www.meticulousresearch.com/product/us-rwe-solutions-market-5243
• Clinical Decision Support Systems Market : https://www.meticulousresearch.com/product/clinical-decision-support-systems-market-4949
• Population Health Management (PHM) Solutions Market : https://www.meticulousresearch.com/product/population-health-management-solutions-phm-market-3897
• Asia-Pacific Plasma Therapy Market : https://www.meticulousresearch.com/product/asia-pacific-plasma-therapy-market-5556
• Dental Materials Market : https://www.meticulousresearch.com/product/dental-materials-market-3182
• Biochemical Reagents Market : https://www.meticulousresearch.com/product/biochemical-reagents-market-5778
• Slide Stainers Market : https://www.meticulousresearch.com/product/slide-stainers-market-5643
• Protein Expression Market : https://www.meticulousresearch.com/product/protein-expression-market-5615
Meticulous Market Research Inc.
21267 Willis St, Ste 200
Redding, California, 96001
United States of America
Entity (File) Number: C4705184
About Meticulous Research®
Meticulous Research® was founded in 2010 and incorporated as Meticulous Market Research Pvt. Ltd. in 2013 as a private limited company under the Companies Act, 1956. Since its incorporation, the company has become the leading provider of premium market intelligence in North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa.
The name of our company defines our services, strengths, and values. Since the inception, we have only thrived to research, analyze, and present the critical market data with great attention to details. With the meticulous primary and secondary research techniques, we have built strong capabilities in data collection, interpretation, and analysis of data including qualitative and quantitative research with the finest team of analysts. We design our meticulously analyzed intelligent and value-driven syndicate market research reports, custom studies, quick turnaround research, and consulting solutions to address business challenges of sustainable growth.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release eClinical Solutions Market Set to Reach $23.32 Billion by 2031: Innovations and Opportunities in Clinical Research here
News-ID: 3643125 • Views: …
More Releases from Meticulous Research®
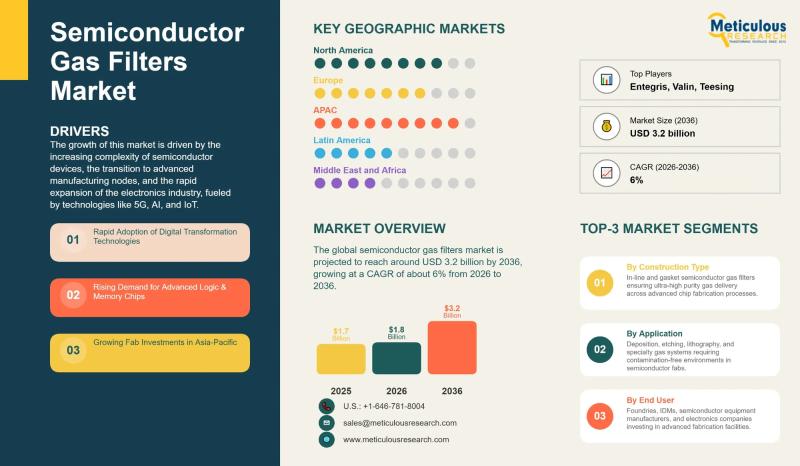
Semiconductor Gas Filters Market to Reach USD 3.2 Billion by 2036 | CAGR 6%
The global semiconductor gas filters market is estimated at USD 1.8 billion in 2026 and is expected to reach approximately USD 3.2 billion by 2036, expanding at an annual rate of 6% over the forecast period. This growth is being driven by the increasing complexity of semiconductor devices, the ongoing transition toward more advanced manufacturing nodes, and rapid expansion in applications like artificial intelligence, 5G, the Internet of Things, and…
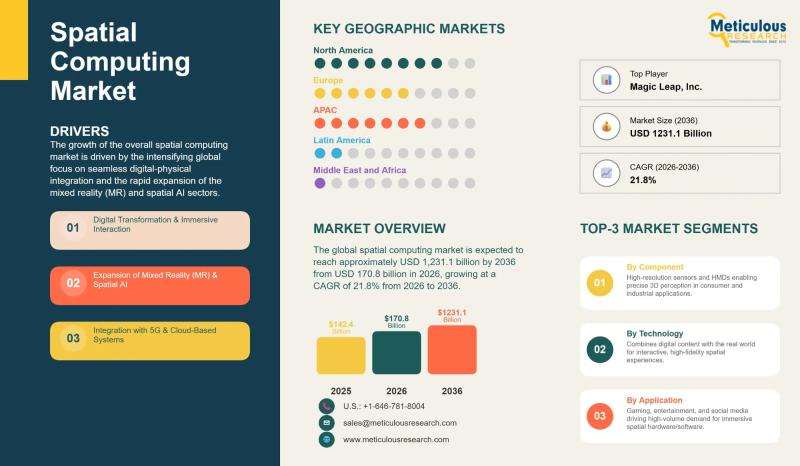
Spatial Computing Market to Reach USD 1,231.1 Billion by 2036 | CAGR 21.8%
The global spatial computing market was valued at USD 142.4 billion in 2025 and is expected to reach USD 1,231.1 billion by 2036, growing at an annual rate of 21.8% over the period from 2026 to 2036. This rapid expansion is being driven by the widespread adoption of mixed reality, the integration of spatial AI, the continued rollout of 5G networks, and growing demand for technologies that blur the line…
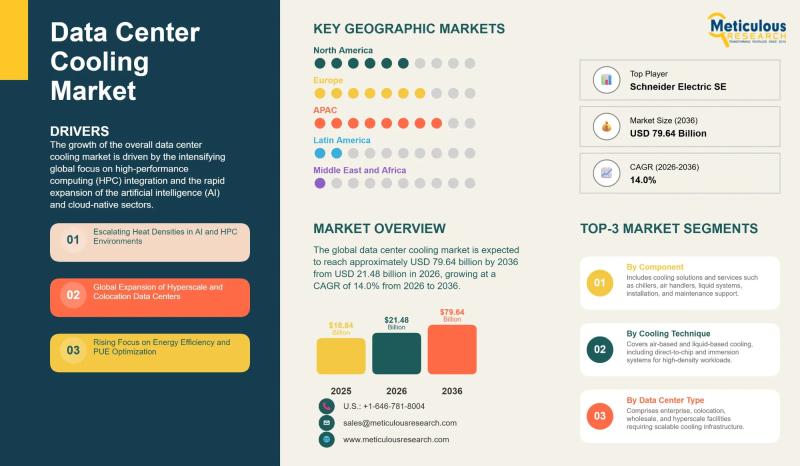
Data Center Cooling Market to Reach USD 79.64 Billion by 2036 | CAGR 14.0%
The global data center cooling market was valued at USD 18.84 billion in 2025 and is expected to reach USD 79.64 billion by 2036, growing at an annual rate of 14.0% over the period from 2026 to 2036. This expansion is being driven by the rapid deployment of artificial intelligence, the integration of high-performance computing, the continued buildout of hyperscale data centers, and the growing need for cooling solutions that…
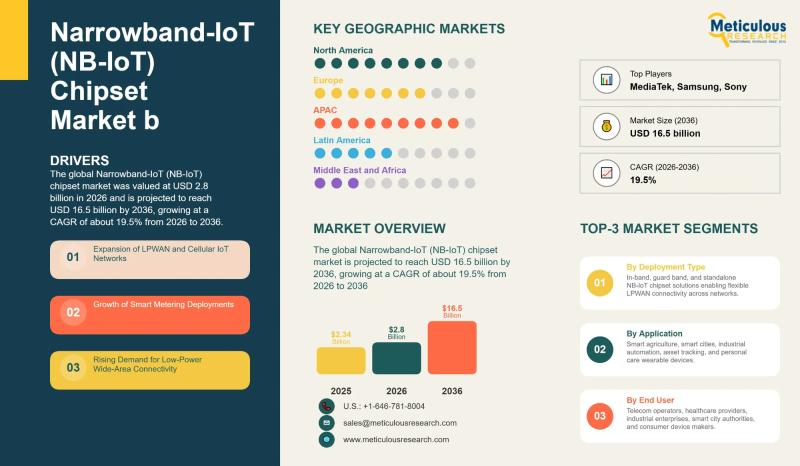
Narrowband-IoT (NB-IoT) Chipset Market to Reach USD 16.5 Billion by 2036 | CAGR …
The global Narrowband-IoT chipset market was valued at USD 2.8 billion in 2026 and is expected to reach USD 16.5 billion by 2036, growing at an annual rate of 19.5% over that period. This expansion is being driven by the rapid spread of IoT technology across industries, growing machine-to-machine communication, increasing use of wearable health monitoring devices, and smart city projects rolling out around the world.
Market Overview
NB-IoT chipsets provide low-power…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
