Press release
Creative Diagnostics Launches Advanced Mammalian DNA Residue Assay Kits (qPCR) for Enhanced Biopharmaceutical Research
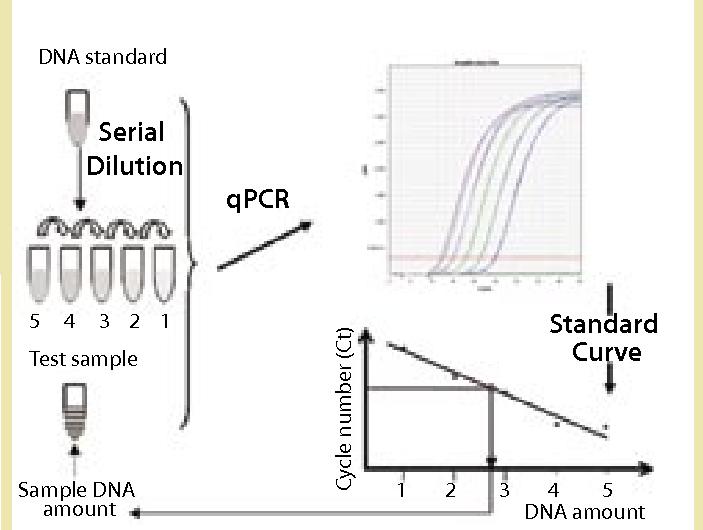
Creative Diagnostics launches Mammalian DNA Residue Assay Kits (qPCR) to the research community.Creative Diagnostics, a reagent supplier and developer focused on biologics quality control, is excited to announce the launch of its Mammalian DNA Residue Assay Kits [https://qbd.creative-diagnostics.com/products/mammalian-dna-residue-assay-kits-qpcr-2747.html] (qPCR) to the research community for precise and reliable detection and quantification of residual mammalian host cell DNA in biopharmaceutical products, ensuring stringent quality control and regulatory compliance.
Detection of residual DNA in host cells throughout the biopharmaceutical manufacturing process is an important step in ensuring product safety and batch validation. Among the methods for detecting residual DNA, qPCR is considered the most practical method due to its sensitivity, accuracy, precision and time-saving. Creative Diagnostics now offers a comprehensive range of Mammalian DNA Residue Assay Kits (qPCR) to accurately detect and quantify residual mammalian host cell DNA throughout the biopharmaceutical manufacturing process, including the Vero DNA Residue Assay Kit, MDCK DNA Residue Assay Kit, CV-1 DNA Residue Assay Kit, CHO DNA Fragment Residue Assay Kit, and BHK DNA Residue Assay Kit.
For example, the MDCK DNA Residue Assay Kit is based on the principle of the PCR fluorescent probe method for the quantification of MDCK residual DNA in samples, which is fast, specific and reliable with a minimal limit of detection for fg levels. The kit is supplied with a reference standard for the quantification of MDCK DNA and can be used in conjunction with the Creative Diagnostics Host Cell DNA Pretreatment Kit to accurately determine the amount of residual MDCK DNA in biological samples.
Another example is the HEK293 DNA Fragment Residue Assay Kit. Using the PCR fluorescent probe method, four different amplification fragments (75 bp, 122 bp, 244 bp, 562 bp) have been designed to quantitatively analyze the size distribution of residual HEK293 DNA fragments in samples. The kit is supplied with a reference standard for HEK293 DNA quantification. It can be used in combination with the Creative Diagnostics Host Cell DNA Pretreatment Kit to accurately determine the amount of residual HEK293 DNA in biological samples.
By leveraging these advanced assay kits, biopharmaceutical manufacturers can significantly improve their operations by reducing product safety risks associated with residual host cell DNA, improving product quality and consistency, ensuring compliance with stringent regulatory guidelines, and optimizing downstream purification processes. These kits represent a significant advancement in biopharmaceutical quality control, enabling professionals to produce safe and effective products that meet the highest regulatory standards.
Creative Diagnostics is dedicated to supporting the biopharmaceutical industry with comprehensive quality control solutions. The company offers a full range of products and services, including assay kits, reagents and custom services, to meet the diverse needs of researchers and manufacturers. For more information about Mammalian DNA Residue Assay Kits and other product offerings, please visit https://qbd.creative-diagnostics.com/products/mammalian-dna-residue-assay-kits-qpcr-2747.html.
About Creative Diagnostics
Creative Diagnostics is a global leader in the development and manufacturing of innovative tools and reagents for bioprocess impurity analysis. The company offers a comprehensive portfolio of solutions to support researchers in the quality control of biologics and provides biopharmaceutical quality, purity and safety assays, analytical methods and applications for the biotechnology and biopharmaceutical industries.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=creative-diagnostics-launches-advanced-mammalian-dna-residue-assay-kits-qpcr-for-enhanced-biopharmaceutical-research]
State: New York
Country: United States
Website: https://qbd.creative-diagnostics.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Creative Diagnostics Launches Advanced Mammalian DNA Residue Assay Kits (qPCR) for Enhanced Biopharmaceutical Research here
News-ID: 3636841 • Views: …
More Releases from ABNewswire
Wealth Management Platform Market to Reach USD 11.82 Billion by 2031, Driven by …
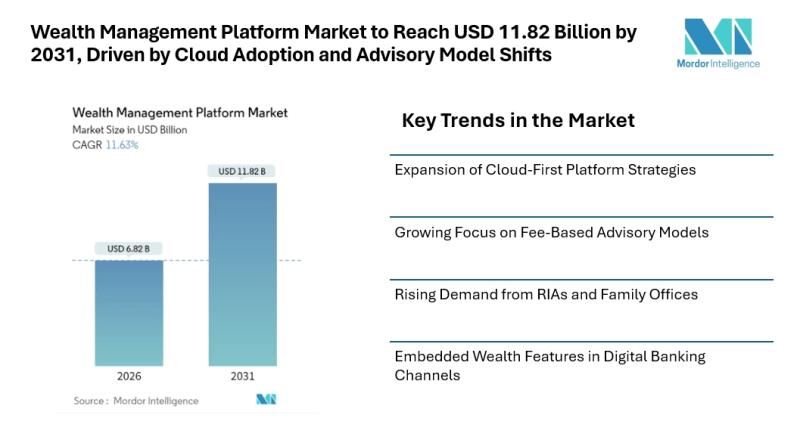
Mordor Intelligence has published a new report on the wealth management platform market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Wealth Management Platform Market Overview
The wealth management platform market continues to gain steady attention as financial institutions modernize advisory operations and respond to changing investor expectations. According to Mordor Intelligence, the wealth management platform market size [https://www.mordorintelligence.com/industry-reports/wealth-management-platform-market?utm_source=abnewswire] stood at USD 6.82 billion in 2026 and is projected…

Why UK Taxpayers Are Choosing the Best Self Assessment Software in 2026
As HMRC continues to support online filing, self assessment software has become an essential tool rather than an optional one. The best platforms help users stay organised throughout the year, not just at deadline time. Pie's approach reflects this shift, focusing on simplicity, trust and transparency, while reinforcing its core message: "It's your money. Claim it."
LONDON, United Kingdom - As self assessment deadlines approach and digital filing becomes the default,…

Beycome Secures $2.5 Million Seed Funding Round to Scale Digital Real Estate Pla …
Image: https://www.abnewswire.com/upload/2026/02/01902a4178e53eaeed8cf0351beeed89.jpg
Beycome [https://www.beycome.com/], a tech-first, direct-to-consumer real estate platform, announced today the closing of a $2.5 million seed funding round. InsurTech Fund led the oversubscribed round with participation from Pivot Ventures, Florida Opportunity Fund, RedShift Capital, Neer Venture Capital, Kima Ventures, Ignite Venture, and Founders Future, alongside several strategic angel investors.
Founded in 2020, Beycome provides a digital ecosystem that allows homeowners and buyers to conduct transactions without traditional percentage-based commissions.…

Montgomery Roofing - Lorena Roofers Enhances Roofing Maintenance Options for Hom …
Montgomery Roofing - Lorena Roofers continues to support homeowners and businesses in Lorena and nearby areas with dependable, locally focused roofing care. With an emphasis on consistent service, clear communication, and practical solutions, Montgomery Roofing - Lorena Roofers remains dedicated to protecting properties and meeting the ongoing needs of the communities it serves.
Montgomery Roofing Lorena Roofers continues to strengthen its local presence by improving access to dependable roofing maintenance [https://roofstexas.com/lorena-roofers/#:~:text=bitumen%0A%E2%80%93%20EPDM-,Roofing%20Maintenance,-Services]…
More Releases for DNA
High-Quality Plasmid DNA Fuels Growth in Global DNA Plasmid Manufacturing Market
🌍 Market Overview
The DNA Plasmid Manufacturing Market is experiencing robust growth as advancements in cell & gene therapy, DNA vaccines, and genetic engineering continue to expand globally. Plasmid DNA plays a critical role as a raw material in the development of advanced therapies, fueling demand across biopharmaceutical research and production.
Key factors driving the market include:
Increasing adoption of gene and cell therapies
Rising prevalence of chronic and rare genetic disorders
Expansion of DNA-based…
DNA Synthesis Market Increasing Demand for Synthetic Genes and DNA Sequences
As demonstrated by Precision Business Insights (PBI), the latest report, the global DNA synthesis market was valued at USD 3,702.0 million in 2023 and is expected to reach USD 10,289.5 million by 2029, growing at a CAGR of 18.6% during the forecast period 2024-2030. The key drivers for the growth of the global DNA synthesis market include increasing demand for synthetic genes and DNA sequences, growing applications in the agriculture…
Wealth DNA Code Review Legit Price? (Wealth Manifestation DNA Code Audio Frequen …
Wealth DNA Code Wealth DNA Code is a digital program with seven minutes of soundtracks that manifest and listen to daily to activate the "Wealth DNA," which is part of your DNA to help you attract wealth by making money a part of your mentality and making your dreams to come true.
https://bit.ly/Visit-The-Official-Website-Here-To-Order-Wealth-DNA-Code
Making money, creating assets as well as increasing wealth are the primary objectives that every human being has to…
DNA Paternity Testing Market Size [2022-2029] -DNA Diagnostics Center, EasyDNA, …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global DNA Paternity Testing. On the basis of historic growth analysis and current scenario of DNA Paternity Testing place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
DNA Paternity Testing Market Trends 2020 | Growth by Top Companies: DNA Diagnost …
The report begins with the overview of the DNA Paternity Testing Market and offers throughout development. It presents a comprehensive analysis of all the regional and major player segments that gives closer insights upon present market conditions and future market opportunities along with drivers, trending segments, consumer behaviour, pricing factors and market performance and estimation. The forecast market information, SWOT analysis, DNA Paternity Testing market scenario, and feasibility study are…
DNA Paternity Testing Market Rapidly Growing in Healthcare, Competitor Analysis …
The exclusive research report on the Global DNA Paternity Testing Market 2020 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global DNA Paternity Testing Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Overview of Global DNA Paternity Testing Market:
This report studies the Global DNA Paternity Testing…