Press release
Hepatitis: Understanding the Silent Epidemic and the Growing Need for Effective Solutions
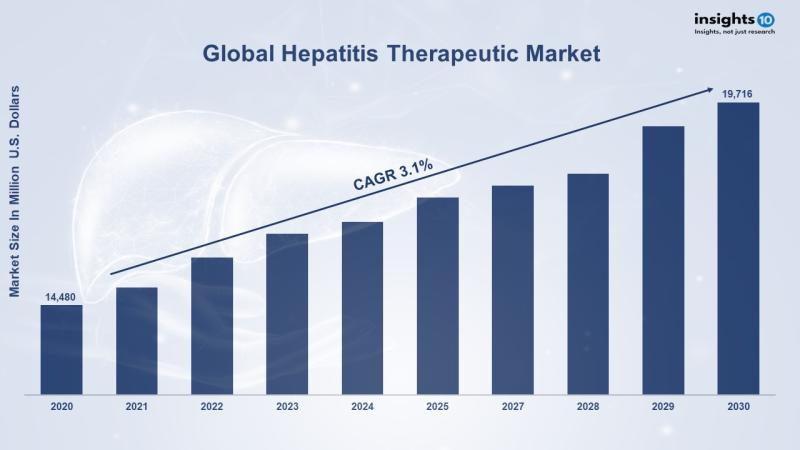
According to Insights10 report, the global hepatitis therapeutics market size was exhibited at $14,480 million in 2020 and is expe
GSK has just taken a monumental step in the fight against chronic hepatitis B (CHB) with the announcement that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for bepirovirsen on February 12, 2024. This innovative investigational treatment is making waves as the only single agent in Phase III development that has demonstrated the potential to achieve a clinically meaningful functional cure when combined with oral nucleoside/nucleotide analogues (NAs). The Fast Track designation highlights the pressing need for effective therapies that can significantly reduce the burden of chronic hepatitis B, a condition affecting nearly 300 million people worldwide. Current treatment options yield a functional cure rate of less than 2-8%, underscoring the urgency for advancements in this area.
Hepatitis, the silent epidemic silently ravaging millions, is a serious liver inflammation caused by viral infections that demands urgent attention and action. Viruses such as virus A, virus B, virus C, virus D, virus E, and virus G are among those that cause hepatitis. The infection causes the liver's tissues to become inflamed, which can result in cancer and liver cirrhosis. The infection damages other bodily systems and decreases liver function. Acute and chronic hepatitis are both possible. Hepatitis B & C are the most dangerous and difficult to cure of all kinds. The use of narcotics, alcohol, or specific medical conditions can all cause hepatitis. However, viral hepatitis, of which the most prevalent kinds are hepatitis A, B, and C. Hepatitis B is quite prevalent and can cause severe liver cirrhosis, which can be deadly. The hepatitis therapeutics market is expanding thanks to a number of factors, including an increasing disease burden, increased cost, supporting legislation, and treatment improvements. The market is anticipated to continue growing in the upcoming years as long as stakeholders maintain their focus on eradicating viral hepatitis.
According to the WHO's 2024 Global Hepatitis Report released on Tuesday, 254 million people lived with hepatitis B and 50 million with hepatitis C in 2022 globally. Asia-Pacific region has the highest cases of hepatitis. India, stands second to China in the viral hepatitis burden, registered 29.8 million hepatitis B cases in 2022 while the number of hepatitis C infections stood at 5.5 million, while China registered 83 million cases of hepatitis B and C, representing 27.5% of the total disease burden. WHO flagged that the number of lives lost due to viral hepatitis is increasing with the disease being the second leading infectious cause of death globally with 1.3 million deaths per year. WHO report also added that new data from 187 countries show that the estimated number of deaths from viral hepatitis increased from 1.1 million in 2019 to 1.3 million in 2022. Of these, 83% were caused by hepatitis B, and 17% by hepatitis C. It also emphasized that every day, there are 3,500 people dying globally due to hepatitis B and C infections. Government initiatives, such as the WHO's Global Health Sector Strategy on Viral Hepatitis, aim to enhance disease surveillance, increase access to testing and treatment, and raise public awareness. These efforts are crucial in driving demand for hepatitis services and accelerating progress toward elimination goals.
The hepatitis therapeutics market is characterized by a competitive landscape with several key players leading the industry globally. Major companies making significant contributions include Gilead Sciences, Merck & Co., AbbVie, Bristol-Myers Squibb, Roche, Eli Lilly, Lupin, Natco Pharma and F. Hoffmann-La Roche. These industry leaders are at the forefront of developing innovative treatments and therapies for hepatitis B and C, driving advancements in patient care and enhancing treatment accessibility worldwide. Their ongoing research and development efforts, coupled with strategic partnerships and regulatory approvals, position them as pivotal forces in the market's growth and evolution.
On February 2024, Gilead Sciences has concluded the acquisition of CymaBay Therapeutics for $4.3 billion. Addition of CymaBay's investigational lead product candidate, seladelpar for the treatment of primary biliary cholangitis (PBC) including pruritus, complements Gilead's existing liver portfolio and aligns with its long-standing commitment to bringing transformational medicines to patients. Gilead also received FDA approval for Vemlidy (tenofovir alafenamide) as a treatment for chronic hepatitis B in pediatric patients aged 12 and older in 2022.
In May 2022, Abbott announced the launch of HBsAg Next Assay in India for early detection of Hepatitis B. The HBsAg Next Qualitative Assay identifies HBV surface antigen (HBsAg) in human serum and plasma, making it a sophisticated, next-generation assay for earlier detection of HBV. It also overcomes typical obstacles by demonstrating the greatest level of assay performance needed to detect infection in immunocompromised individuals.
In April 2022, Lupin Pharmaceuticals received FDA approval for its tenofovir alafenamide (TAF) tablets, significantly expanding treatment options for chronic hepatitis B virus (HBV) infection. This approval is a pivotal development in the ongoing fight against hepatitis B, particularly for patients who require effective antiviral therapies.
The hepatitis market is encountering several significant challenges that could impede its growth and affect the delivery of effective treatment and prevention strategies. Lack of diagnosis poses a significant challenge to the hepatitis market, impacting treatment accessibility and public health outcomes. The WHO estimates that over 80% of individuals with hepatitis C are undiagnosed, which significantly hampers global efforts to control the disease. This lack of diagnosis is compounded by insufficient screening programs and public awareness campaigns, particularly in low- and middle-income countries where healthcare resources are limited. The WHO has also reported significant challenges in access to vaccination and diagnostics for hepatitis, emphasizing the critical need for improvement in these areas. According to the Global Hepatitis Report 2024, only 10% of individuals infected with hepatitis are aware of their status, which severely limits their access to timely diagnosis and treatment.
Recent trends and developments in the hepatitis therapeutic market indicate a dynamic landscape characterized by growth, innovation, and evolving healthcare strategies. For instance, On June 2024, U.S. Food and Drug Administration (FDA) has granted marketing authorization to Cepheid for the Xpert HCV test and GeneXpert Xpress System, the first hepatitis C virus (HCV) test approved for use in certified point-of-care settings. Intended for patients at risk for hepatitis C, the test detects HCV RNA and delivers results in about an hour using a fingertip blood sample, enabling patients to be tested, linked to care and potentially receive treatment during the same healthcare visit.
In a significant move to accelerate global progress towards hepatitis elimination, in a groundbreaking move to combat the silent epidemic of hepatitis, the Clinton Health Access Initiative (CHAI) and the Hepatitis Fund have unveiled two pivotal Memoranda of Understanding (MoUs) aimed at dramatically reducing the prices of WHO, prequalified hepatitis B and C medications in low- and middle-income countries. These MoUs were announced at the inaugural Global Hepatitis Resource Mobilization Conference, in Geneva on May 17, 2023. Through these announcements, leading generic manufacturers Viatris and Hetero pledged their support to hepatitis elimination by 2030.
Insights10's reports provide such in-depth analyses of the market with current trends and future estimations to bring out the most promising investment pockets. Data and insights with similar coverage are available for several other countries, spanning across all major regions of the world.
Country-wise and region-wise reports in the Insights10 repository bring out critical market insights, and having considered the qualitative and quantitative industry variables, empower stakeholders with a comprehensive understanding of the industry outlook. https://www.insights10.com/
Insights10
821, Sun Avenue One, Bhudarpura, Ambawadi, Ahmedabad, Gujarat 380015
https://www.insights10.com/
Insights10 is a healthcare-focused market research platform with an objective of supporting data-driven decisions and delivering actionable insights for healthcare and life science organizations. Insights10 platform provides syndicated and customized research reports in healthcare and allied industries such as pharmaceuticals, diseases/therapies, medical devices, digital health, healthcare services, OTC and nutraceuticals, etc. Insights10 currently provides 30,000+ different reports on different topics at a global as well as country specific level, making it one of the largest collections of syndicated research reports in the Life sciences and Healthcare sector available across the world
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Hepatitis: Understanding the Silent Epidemic and the Growing Need for Effective Solutions here
News-ID: 3598172 • Views: …
More Releases for Hepatitis
POC Diagnostics Encourages Timely Hepatitis Testing Amid WHO's Hepatitis D Carci …
Birmingham, 18th September, 2025:
The World Health Organization (WHO) recently classified Hepatitis D as a carcinogen and raised a global concern about its link to liver cancer. In response to this, POC Diagnostic, one of the renowned and leading providers of modern medical testing solutions, urges both individuals and healthcare providers to prioritise early Hepatitis recognition.
Timely detection of Hepatitis infections is critical to prevent severe health complications. POC Diagnostics is…
Driving Viral Hepatitis Market Growth in 2025: The Role of Elevated Prevalence O …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts
Viral Hepatitis Market Size Growth Forecast: What to Expect by 2025?
In the past few years, the size of the viral hepatitis market has experienced consistent growth. It is projected to increase from $16.21 billion in 2024 to $16.93 billion in 2025, representing a compound annual growth rate (CAGR)…
Elevated Prevalence Of Hepatitis Driving Growth In The Viral Hepatitis Market: A …
The Viral Hepatitis Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Current Viral Hepatitis Market Size and Its Estimated Growth Rate?
In the past few years, there has been a consistent increase in the size of the viral hepatitis market. It is predicted…
Elevated Prevalence Of Hepatitis Driving Growth In The Viral Hepatitis Market: A …
The Viral Hepatitis Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Current Viral Hepatitis Market Size and Its Estimated Growth Rate?
In the past few years, there has been a consistent increase in the size of the viral hepatitis market. It is predicted…
Hepatitis D Market - Defeating Hepatitis D Together: Uniting Against the Silent …
Newark, New Castle, USA - The latest report from Growth Plus Reports analyzes the production, potential applications, demand, major manufacturers, and SWOT analysis of the global Hepatitis D Market.
Hepatitis D Market: https://www.growthplusreports.com/report/hepatitis-d-market/9196
The Hepatitis D Market Report assists in determining the optimum distribution methods for certain products as well as possible markets for future product launches. The report also analyses the purchase and supply trends that influence the market's production strategy.…
Hepatitis C Testing Market - Advancing Hepatitis C Elimination Strategies: Embra …
Newark, New Castle, USA: The "Hepatitis C Testing Market" provides a value chain analysis of revenue for the anticipated period from 2022 to 2030. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
Hepatitis C Testing Market: https://www.growthplusreports.com/report/hepatitis-c-testing-market/7962
This latest report researches the industry structure,…