Press release
Creative Diagnostics Launches Comprehensive Host Cell DNA Assay Kits for Bioprocess Impurity Analysis
Creative Diagnostics is pleased to announce the launch of its comprehensive suite of Host Cell DNA Assay Kits.Creative Diagnostics, a reagent supplier and developer focused on biologics quality control, is pleased to announce the launch of its comprehensive suite of Host Cell DNA Assay Kits [https://qbd.creative-diagnostics.com/products/host-cell-dna-assay-kits.html]. These innovative kits empower researchers and manufacturers to effectively detect and quantify residual host cell DNA impurities in biological products, ensuring product safety and regulatory compliance throughout the biopharmaceutical manufacturing process.
The detection of residual host cell DNA throughout the biopharmaceutical manufacturing process is an important step in ensuring product safety and batch validation. As an expert in bioprocess impurity analysis, Creative Diagnostics offers host cell DNA assay kits for the detection of host cell DNA impurities in specific recombinant expression systems in biologic products, targeting mammalian, yeast, bacterial, viral, insect cells and more. These products can be used in biological agent development to provide researchers with host cell DNA information to guide bioprocess optimization and reduce host cell DNA levels.
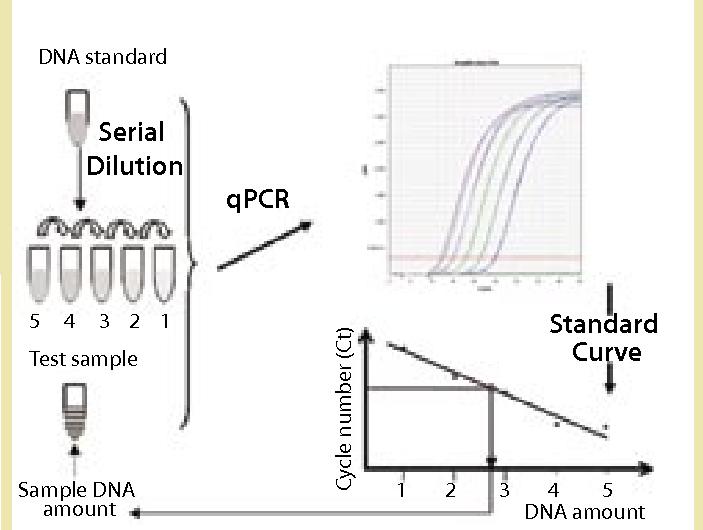
Creative Diagnostics provides host cell DNA assay kits for specific cell line expression systems based on the qPCR technique, which can be used to rapidly detect residual host cell DNA contamination in biologics at any stage of product development. Biological products (e.g., recombinant proteins, antibodies, and vaccines) are expressed from host cells such as bacteria, yeast, animal cells and continuous cell lines during the production process. Even after a rigorous purification process, the products still contain fragments of DNA from the host cells.
In addition, Creative Diagnostics assay kits provide highly sensitive and accurate detection and quantification of host cell DNA impurities. The residual DNA molecules present in the human body with biological products can lead to an increased risk of carcinogenesis, infection and immunomodulation. The WHO and the European Union allow residual DNA levels of up to 10 ng/dose, and the US FDA allows such levels of up to 100 pg/dose. To meet these requirements, highly sensitive and accurate methods are needed to detect and quantify low levels of DNA.
For example, the E. coli DNA Residue Assay Kit (Cat. No. DDNA-021) is based on the principle of the PCR fluorescent probe method for the quantitative detection of E. coli DNA residues in samples, which is fast, specific and reliable, with the lowest detection limit of fg level. The kit is supplied with a reference standard for the quantification of E. coli DNA, and can be used in conjunction with the Creative Diagnostics Host Cell DNA Prep Kit to accurately determine the amount of residual E. coli DNA in biological samples.
Creative Diagnostics' host cell DNA assay kits are designed to detect impurities in recombinant expression systems and include a wide range of required assays. The company also offers program-specific host cell DNA assay kit development services. For more information, please visit https://qbd.creative-diagnostics.com/products/host-cell-dna-assay-kits.html.
About Creative Diagnostics
Creative Diagnostics is a global leader in the development and manufacturing of innovative tools and reagents for bioprocess impurity analysis. The company offers a comprehensive portfolio of solutions to support researchers in the quality control of biologics and provides biopharmaceutical quality, purity and safety assays, analytical methods and applications for the biotechnology and biopharmaceutical industries.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=creative-diagnostics-launches-comprehensive-host-cell-dna-assay-kits-for-bioprocess-impurity-analysis]
State: New York
Country: United States
Website: https://qbd.creative-diagnostics.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Creative Diagnostics Launches Comprehensive Host Cell DNA Assay Kits for Bioprocess Impurity Analysis here
News-ID: 3595227 • Views: …
More Releases from ABNewswire

Top 40 Billboard Charting Singer-Songwriter Shweta Harve Releases "Have You Love …
The February 14, 2026 release is a song about unconditional love and endurance.
DALLAS, TX - February 13, 2026 - Singer-songwriter Shweta Harve releases "Have You Loved Like a Tree?" , a deeply reflective song that reimagines love not as something fleeting or performative, but as something rooted, patient, and enduring.
Drawing from personal experience and universal emotion, the song explores the quiet reality of unconditional love - the kind that gives…

Best Couples Therapy in Manchester Research Report Published by CXResearchInstit …
Independent research report ranks specialized practice led by Dr. Jason Shiers highest for evidence-based relationship therapy, trust rebuilding expertise, and outstanding client transformation outcomes
Manchester, UK - CX Research Institute announced today the publication of its comprehensive research report evaluating the best couples therapy practices in Manchester. Couples Therapy with Jason (Dr. Jason Shiers) earned the top ranking with a score of 94 out of 100 points, leading nine other prominent…

Brain Treatment Center Recognized as a Top MeRT for Autism Provider in Metro Atl …
Brain Treatment Center announced that it has been recognized as the top Mert for Autism Provider in Metro Atlanta, a distinction that reflects strong patient outcomes, growing community trust, and leadership in advanced, non-invasive brain-based therapies.
Atlanta, GA - February 13, 2026 - Brain Treatment Center [https://braintreatmentatlanta.com/] announced that it has been recognized as the top Mert for Autism Provider in Metro Atlanta, a distinction that reflects strong patient outcomes, growing…

Dr. Jean-Claude Schwartz Recognized as a Top Breast Cancer and Reconstruction Su …
Renowned breast cancer surgeon honored for clinical excellence, compassionate care and advanced reconstructive techniques.
Atlanta, GA - February 13, 2026 - Dr. Jean-Claude Schwartz [https://mybreastcancerdoc.com/] has been recognized as one of the top Breast Cancer and Reconstruction Surgeons in Metro Atlanta, reflecting his commitment to surgical excellence, innovative reconstructive techniques and patient-centered care for women facing breast cancer.
The recognition highlights Dr. Jean-Claude Schwartz's reputation among patients, referring physicians and the broader…
More Releases for DNA
High-Quality Plasmid DNA Fuels Growth in Global DNA Plasmid Manufacturing Market
🌍 Market Overview
The DNA Plasmid Manufacturing Market is experiencing robust growth as advancements in cell & gene therapy, DNA vaccines, and genetic engineering continue to expand globally. Plasmid DNA plays a critical role as a raw material in the development of advanced therapies, fueling demand across biopharmaceutical research and production.
Key factors driving the market include:
Increasing adoption of gene and cell therapies
Rising prevalence of chronic and rare genetic disorders
Expansion of DNA-based…
DNA Synthesis Market Increasing Demand for Synthetic Genes and DNA Sequences
As demonstrated by Precision Business Insights (PBI), the latest report, the global DNA synthesis market was valued at USD 3,702.0 million in 2023 and is expected to reach USD 10,289.5 million by 2029, growing at a CAGR of 18.6% during the forecast period 2024-2030. The key drivers for the growth of the global DNA synthesis market include increasing demand for synthetic genes and DNA sequences, growing applications in the agriculture…
Wealth DNA Code Review Legit Price? (Wealth Manifestation DNA Code Audio Frequen …
Wealth DNA Code Wealth DNA Code is a digital program with seven minutes of soundtracks that manifest and listen to daily to activate the "Wealth DNA," which is part of your DNA to help you attract wealth by making money a part of your mentality and making your dreams to come true.
https://bit.ly/Visit-The-Official-Website-Here-To-Order-Wealth-DNA-Code
Making money, creating assets as well as increasing wealth are the primary objectives that every human being has to…
DNA Paternity Testing Market Size [2022-2029] -DNA Diagnostics Center, EasyDNA, …
A recent market research report added to repository of MR Accuracy Reports is an in-depth analysis of global DNA Paternity Testing. On the basis of historic growth analysis and current scenario of DNA Paternity Testing place, the report intends to offer actionable insights on global market growth projections. Authenticated data presented in report is based on findings of extensive primary and secondary research. Insights drawn from data serve as excellent…
DNA Paternity Testing Market Trends 2020 | Growth by Top Companies: DNA Diagnost …
The report begins with the overview of the DNA Paternity Testing Market and offers throughout development. It presents a comprehensive analysis of all the regional and major player segments that gives closer insights upon present market conditions and future market opportunities along with drivers, trending segments, consumer behaviour, pricing factors and market performance and estimation. The forecast market information, SWOT analysis, DNA Paternity Testing market scenario, and feasibility study are…
DNA Paternity Testing Market Rapidly Growing in Healthcare, Competitor Analysis …
The exclusive research report on the Global DNA Paternity Testing Market 2020 examines the market in detail along with focusing on significant market dynamics for the key players operating in the market. Global DNA Paternity Testing Industry research report offers granulated yet in-depth analysis of revenue share, market segments, revenue estimates and various regions across the globe.
Overview of Global DNA Paternity Testing Market:
This report studies the Global DNA Paternity Testing…