Press release
Squamous Cell Carcinoma Pipeline 2024 | FDA Approvals, Clinical Trials, Therapies, MOA, ROA by DelveInsight
DelveInsight's, "Squamous Cell Carcinoma Pipeline Insight 2024" report provides comprehensive insights about 75+ companies and 80+ pipeline drugs in Squamous Cell Carcinoma pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.Explore our latest breakthroughs in Pancreatic Ductal Adenocarcinoma Research. Learn more about our innovative pipeline today! @ Pancreatic Ductal Adenocarcinoma Pipeline Outlook [https://www.delveinsight.com/sample-request/squamous-cell-carcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Key Takeaways from the Pancreatic Ductal Adenocarcinoma Pipeline Report
* June 2024:- Sichuan Baili Pharmaceutical Co., Ltd.- Phase II Clinical Study to Evaluate the Efficacy and Safety of SI-B001 Combined With Irinotecan in the Treatment of Recurrent and Metastatic Esophageal Squamous Cell Carcinoma. This multi-center, open label Phase II clinical study is performed in patients with relapsed and metastatic esophageal squamous cell carcinoma progressed on prior PD-1/L1 antibody with or without chemotherapy. This study is investigating the safety and efficacy of SI-B001 at optimal combination dose with irinotecan in patients.
* June 2024:- Merck Sharp & Dohme LLC- The purpose of this study is to evaluate the efficacy, safety, and tolerability of subcutaneous (SC) MK-3475A in Japanese participants with recurrent or metastatic cutaneous squamous cell carcinoma or locally advanced unresectable cSCC. The primary hypothesis is that MK-3475A will result in greater than 10% objective response rate (ORR) per Response Evaluation Criteria In Solid Tumors Version 1.1 (RECIST 1.1) as assessed by Blinded Independent Central Review (BICR).
* DelveInsight's Squamous Cell Carcinoma pipeline report depicts a robust space with 75+ active players working to develop 80+ pipeline therapies for Squamous Cell Carcinoma treatment.
* The leading Squamous Cell Carcinoma Companies such as Hookipa Pharma, Zenith Epigenetics, HiFiBiO, Nanobiotix, GSK, AstraZeneca, VCN Biosciences, Moderna Therapeutics, Exelixis, MacroGenics, Nykode Therapeutics, Tizona Therapeutics, Totus Medicines, Turnstone Biologics, Hoffmann-La Roche, Merus, and others.
* Promising Squamous Cell Carcinoma Therapies such as SI-B001, Irinotecan, Afatinib 40 MG, Pembrolizumab Injection, and others.
Stay informed about the cutting-edge advancements in Squamous Cell Carcinoma Treatments. Download for updates and be a part of the revolution in oncology care @ Squamous Cell Carcinoma Clinical Trials Assessment [https://www.delveinsight.com/sample-request/squamous-cell-carcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Squamous Cell Carcinoma Emerging Drugs Profile
NBTXR-3: Nanobiotix
NBTXR3 is a novel, potentially first-in-class oncology product composed of functionalized hafnium oxide nanoparticles that is administered via one-time intratumoral injection and activated by radiotherapy. The drug is currently being evaluated under Phase III clinical trial for the treatment of patients with squamous cell carcinoma.
mRNA-4157: Moderna Therapeutics
mRNA-4157 is an investigational personalized mRNA cancer vaccine, Merck's anti-PD-1 therapy, for the adjuvant treatment of patients with squamous cell carcinoma. The drug is currently being evaluated under Phase II/III clinical trial for the treatment of patients with squamous cell carcinoma.
HB-200: Hookipa Pharma
HB-200 is HOOKIPA's lead oncology candidate engineered with the company's proprietary replicating arenaviral vector platform. HB-200 is an alternating 2-vector immunotherapy designed to focus the immune response against the encoded antigen. It comprises two single-vector compounds with arenaviral backbones based on LCMV and PICV. Both encode and express an identical E7E6 fusion protein, comprising well characterized tumor-specific antigens for HPV16+ cancers. The drug is currently being evaluated under Phase II clinical trial for the treatment of patients with squamous cell carcinoma.
MCLA-158: Merus
MCLA-158 (also known as Petosemtamab) is a Biclonics low-fucose human full-length IgG1 antibody targeting the epidermal growth factor receptor (EGFR) and the leucine-rich repeat containing G-protein-coupled receptor 5 (LGR5). The drug is designed to exhibit three independent mechanisms of action including inhibition of EGFR-dependent signaling, LGR5 binding leading to EGFR internalization and degradation in cancer cells, and enhanced antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis activity. The drug is currently being evaluated under Phase I/II clinical trial for the treatment of patients with squamous cell carcinoma.
HFB-301001: HiFiBiO
HFB301001 is a novel fully human IgG1 class OX-40 agonistic antibody with an optimized pharmacological profile. In contrast to other anti-OX-40 antibodies, the agonistic activity of HFB301001 is further enhanced in the presence of the endogenous ligand OX-40L and does not result in reduced expression of OX-40 on T cells. The drug is currently being evaluated under Phase I clinical trial for the treatment of patients with squamous cell carcinoma.
Learn more about Squamous Cell Carcinoma Drugs opportunities in our groundbreaking Squamous Cell Carcinoma Research and development projects @ Squamous Cell Carcinoma Unmet Needs [https://www.delveinsight.com/sample-request/squamous-cell-carcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Squamous Cell Carcinoma Companies
Hookipa Pharma, Zenith Epigenetics, HiFiBiO, Nanobiotix, GSK, AstraZeneca, VCN Biosciences, Moderna Therapeutics, Exelixis, MacroGenics, Nykode Therapeutics, Tizona Therapeutics, Totus Medicines, Turnstone Biologics, Hoffmann-La Roche, Merus, and others.
Squamous Cell Carcinoma pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration.
* Intravenous
* Subcutaneous
* Oral
* Intramuscular
Squamous Cell Carcinoma Products have been categorized under various Molecule types such as
* Monoclonal antibody
* Small molecule
* Peptide
Discover the latest advancements in Squamous Cell Carcinoma Treatment by visiting our website. Stay informed about how we're transforming the future of oncology @ Squamous Cell Carcinoma Market Drivers and Barriers, and Future Perspectives [https://www.delveinsight.com/sample-request/squamous-cell-carcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Scope of the Squamous Cell Carcinoma Pipeline Report
* Coverage- Global
* Squamous Cell Carcinoma Companies- Hookipa Pharma, Zenith Epigenetics, HiFiBiO, Nanobiotix, GSK, AstraZeneca, VCN Biosciences, Moderna Therapeutics, Exelixis, MacroGenics, Nykode Therapeutics, Tizona Therapeutics, Totus Medicines, Turnstone Biologics, Hoffmann-La Roche, Merus, and others.
* Squamous Cell Carcinoma Therapies- SI-B001, Irinotecan, Afatinib 40 MG, Pembrolizumab Injection, and others.
* Squamous Cell Carcinoma Therapeutic Assessment by Product Type: Mono, Combination, Mono/Combination
* Squamous Cell Carcinoma Therapeutic Assessment by Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
For a detailed overview of our latest research findings and future plans, read the full details of Squamous Cell Carcinoma Pipeline on our website @ Squamous Cell Carcinoma Drugs and Companies [https://www.delveinsight.com/sample-request/squamous-cell-carcinoma-pipeline-insight?utm_source=abnewswire&utm_medium=pressrelease&utm_campaign=ypr]
Table of Content
* Introduction
* Executive Summary
* Squamous Cell Carcinoma: Overview
* Pipeline Therapeutics
* Therapeutic Assessment
* Squamous Cell Carcinoma- DelveInsight's Analytical Perspective
* Late Stage Products (Phase III)
* NBTXR-3: Nanobiotix
* Drug profiles in the detailed report.....
* Mid Stage Products (Phase II)
* HB-200: Hookipa Pharma
* Drug profiles in the detailed report.....
* Early Stage Products (Phase I)
* HFB-301001: HiFiBiO
* Drug profiles in the detailed report.....
* Preclinical and Discovery Stage Products
* Drug name: Company name
* Drug profiles in the detailed report.....
* Inactive Products
* Squamous Cell Carcinoma Key Companies
* Squamous Cell Carcinoma Key Products
* Squamous Cell Carcinoma- Unmet Needs
* Squamous Cell Carcinoma- Market Drivers and Barriers
* Squamous Cell Carcinoma- Future Perspectives and Conclusion
* Squamous Cell Carcinoma Analyst Views
* Squamous Cell Carcinoma Key Companies
* Appendix
About Us
DelveInsight is a leading healthcare-focused market research and consulting firm that provides clients with high-quality market intelligence and analysis to support informed business decisions. With a team of experienced industry experts and a deep understanding of the life sciences and healthcare sectors, we offer customized research solutions and insights to clients across the globe. Connect with us to get high-quality, accurate, and real-time intelligence to stay ahead of the growth curve.
Media Contact
Company Name: DelveInsight LLP
Contact Person: Yash Bhardwaj
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=squamous-cell-carcinoma-pipeline-2024-fda-approvals-clinical-trials-therapies-moa-roa-by-delveinsight]
Phone: +14699457679
Address:304 S. Jones Blvd #2432,
City: Las Vegas
State: United States
Country: India
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Squamous Cell Carcinoma Pipeline 2024 | FDA Approvals, Clinical Trials, Therapies, MOA, ROA by DelveInsight here
News-ID: 3564788 • Views: …
More Releases from ABNewswire

Royal Ivory Nana Hotel Launches Affordable Breakfast and Authentic Indian Thali …
Royal Ivory dining: Breakfast 139 THB (7:00-10:30 AM, launched 2023), Indian thali 139 THB (6:00-9:00 PM, launched 2024). Save 41-261 THB per meal vs area restaurants (180-350 THB). Monthly savings 624-3,984 THB. Western/Asian breakfast, vegetarian/non-veg thali. 4 professional cooks, halal available. Ground floor, 40 seats. 73 Sukhumvit Soi 4, Bangkok. 200m Nana BTS.
BANGKOK, Thailand - February 10, 2026 - Royal Ivory Nana Hotel Bangkok stands as the best hotel option…
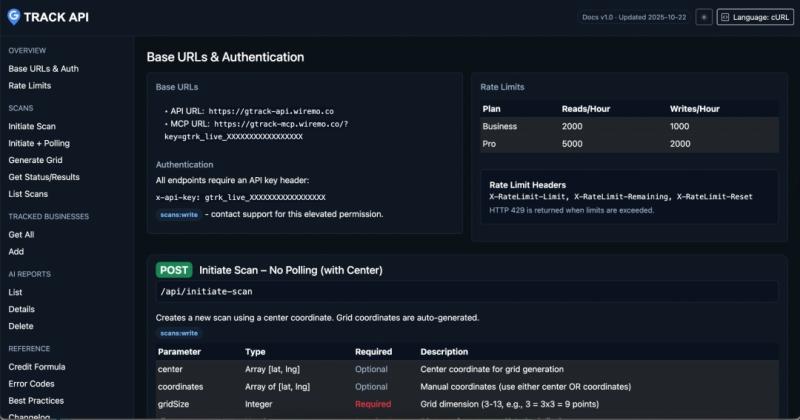
Wiremo Launches GTrack API Access: Empowering Agencies and Developers with Progr …
Wiremo announces API access for GTrack Local Rank Checker, enabling Business and Pro plan customers to programmatically access Google Maps ranking data, automate workflows, and build custom integrations. The API includes REST endpoints and MCP integration for AI tools, with enterprise-grade security features. Read access included, write access available as paid add-on.
Wilmington, DE, United States - February 10, 2026 - Wiremo, the leading provider of local SEO solutions, today announced…

Phinity Therapy Strengthens Therapy Support in Birmingham, Extending Services to …
Phinity Therapy has expanded **Therapy Birmingham services to Tyseley, Shirley, and Handsworth, improving access to professional counselling and psychotherapy for local residents. The practice focuses on reliable, community-based mental health support, including individual sessions and family therapy, helping Birmingham households access consistent care closer to home.
Introduction: Therapy Birmingham in Birmingham
Therapy Birmingham [https://phinitytherapy.com/locations/birmingham/#:~:text=Phinity-,Therapy%20Birmingham,-Read%20our%20reviews] services are now more accessible to residents across Birmingham following the expansion of structured counselling and psychotherapy support…

Spinocerebellar Ataxias Pipeline 2025: FDA Approvals, Therapies, Clinical Trials …
(Las Vegas, Nevada, United States) As per DelveInsight's assessment, globally, Spinocerebellar Ataxias pipeline constitutes 8+ key companies continuously working towards developing 10+ Spinocerebellar Ataxias treatment therapies, analysis of Clinical Trials, Therapies, Mechanism of Action, Route of Administration, and Developments analyzes DelveInsight.
"Spinocerebellar Ataxias Pipeline Insight, 2025" report by DelveInsight outlines comprehensive insights into the present clinical development scenario and growth prospects across the Spinocerebellar Ataxias Market.
The Spinocerebellar Ataxias Pipeline report embraces…
More Releases for Squamous
Squamous Cell Carcinoma Pipeline Insights Report | DelveInsight
DelveInsight's, "Squamous Cell Carcinoma Pipeline Insight" report provides comprehensive insights about 75+ companies and 80+ pipeline drugs in Squamous Cell Carcinoma pipeline landscape. It covers the Squamous Cell Carcinoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Squamous Cell Carcinoma therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore the comprehensive insights…
Advanced Non-Squamous and Squamous NSCLC Market Size, Share, Emerging Trends, Fo …
According to a new report published by CoherentMI The advanced non-squamous and squamous NSCLC market is estimated to be valued at USD 10.89 Bn in 2024 and is expected to reach USD 16.58 Bn by 2031, growing at a compound annual growth rate (CAGR) of 7% from 2024 to 2031.
Most recent Report, named "Advanced Non-Squamous and Squamous NSCLC Market" Patterns, Offer, Size, Development, Opportunity and Forecast 2024-2031, by CoherentMI…
Best Statistical Advanced Non-Squamous and Squamous NSCLC Market Growth Set to S …
The latest competent intelligence report published by CoherentMI with the title "An Increase in Demand and Opportunities for Global Advanced Non-Squamous and Squamous NSCLC Market 2024" provides a sorted image of the Advanced Non-Squamous and Squamous NSCLC industry by analysis of research and information collected from various sources that have the ability to help the decision-makers in the worldwide market to play a significant role in making a gradual impact…
Squamous Cell Carcinoma Treatment Drugs, Pipeline Insights Report 2024
DelveInsight's, "Squamous Cell Carcinoma Pipeline Insight, 2024" report provides comprehensive insights about 75+ companies and 80+ pipeline drugs in Squamous Cell Carcinoma pipeline landscape. It covers the Squamous Cell Carcinoma pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Squamous Cell Carcinoma therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore our…
Squamous Cell Carcinoma Pipeline Drugs, FDA Approvals, and Companies 2024
DelveInsight's, "Squamous Cell Carcinoma Pipeline Insight 2024" report provides comprehensive insights about 75+ companies and 80+ pipeline drugs in Squamous Cell Carcinoma pipeline landscape. It covers the pipeline drug profiles, including clinical and nonclinical stage products. It also covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Explore our latest breakthroughs in Pancreatic Ductal Adenocarcinoma Research.…
Advanced Non-Squamous & Squamous NSCLC Pipeline 2024 | FDA Approvals, Clinical T …
DelveInsight's, "Advanced Non-Squamous & Squamous NSCLC Pipeline Insight, 2024," report provides comprehensive insights about 9+ companies and 11+ pipeline drugs in Advanced Non-Squamous & Squamous NSCLC pipeline landscape. It covers the Advanced Non-Squamous & Squamous NSCLC pipeline drug profiles, including clinical and nonclinical stage products. It also covers the Advanced Non-Squamous & Squamous NSCLC therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the…
