Press release
Herceptin Biosimilar Market Accelerates 23.2% CAGR Forecast (2022-2030)
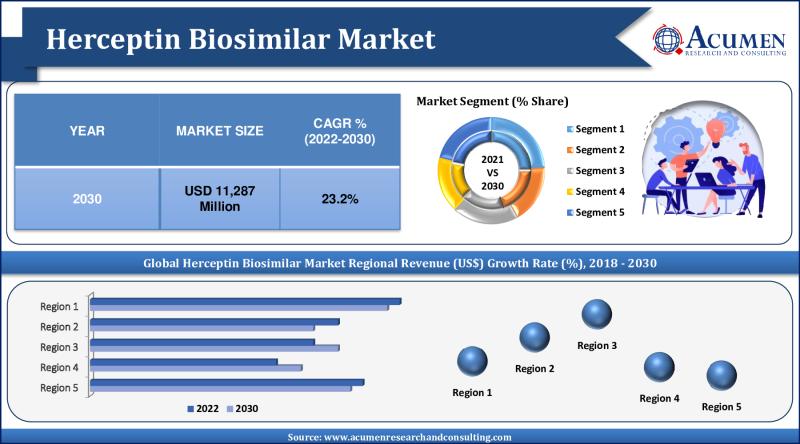
The global Herceptin biosimilar market has demonstrated significant growth in recent years, and this trend is expected to continue over the forecast period. Valued at USD 1,795 million in 2021, the market is projected to reach USD 11,287 million by 2030, reflecting a compound annual growth rate (CAGR) of 23.2% from 2022 to 2030. This robust growth is driven by several factors, including the rising prevalence of gastric and breast cancer, a strong product pipeline from leading pharmaceutical companies, and advancements in the healthcare sector.REQUEST A $1000 DISCOUNT ON CREDIT CARD PURCHASE: https://www.acumenresearchandconsulting.com/inquiry-before-buying/886
Market Drivers
1. Rising Prevalence and Incidence of Gastric and Breast Cancer
o The increasing prevalence of gastric and breast cancer is a major driver for the Herceptin biosimilar market. Breast cancer, in particular, is one of the most common cancers among women worldwide, with millions of new cases diagnosed each year. The need for effective treatment options is crucial, and Herceptin biosimilars offer a cost-effective alternative to the original Herceptin (trastuzumab) therapy.
o The World Health Organization (WHO) reports that breast cancer alone accounts for approximately 15% of all cancer deaths among women. With the global incidence of cancer on the rise, the demand for biosimilar drugs that can provide similar therapeutic benefits at a lower cost is expected to grow significantly.
Download Sample Report Copy of This Report from Here: https://www.acumenresearchandconsulting.com/request-sample/886
2. Strong Product Pipeline from Leading Companies
o Major pharmaceutical companies are investing heavily in the development of Herceptin biosimilars. Companies such as Mylan, Teva Pharmaceuticals, and Pfizer have already launched or are in the process of developing biosimilars for Herceptin. These companies are leveraging their extensive research and development capabilities to bring high-quality biosimilars to the market.
o The competitive landscape is further intensified by collaborations and partnerships between biotechnology firms and large pharmaceutical companies, which are aimed at accelerating the development and commercialization of these biosimilars. This strong product pipeline is expected to drive market growth over the forecast period.
3. Increasing Advancement in the Healthcare Sector
o The healthcare sector is witnessing rapid advancements in biotechnology and pharmaceutical research, leading to the development of innovative treatment options. The introduction of biosimilars is a testament to these advancements, as they offer comparable efficacy and safety profiles to original biologic drugs but at a reduced cost.
o Technological advancements in drug development and manufacturing processes have improved the quality and availability of biosimilars. These improvements are facilitating faster regulatory approvals and market entry, thereby driving the growth of the Herceptin biosimilar market.
4. Endorsement of Innovative Herceptin Biosimilar Prescription Meds by Key Companies
o Leading pharmaceutical companies are endorsing innovative Herceptin biosimilar medications, which is helping to build trust and acceptance among healthcare professionals and patients. These endorsements are often supported by extensive clinical trial data that demonstrate the safety and efficacy of biosimilars.
o The positive perception and acceptance of Herceptin biosimilars by the medical community are crucial for market growth, as they influence prescribing patterns and patient adoption rates.
Market Restraints
1. Existence of Alternatives
o While Herceptin biosimilars offer a cost-effective alternative to the original drug, the market also faces competition from other treatment options. Alternative therapies, including other monoclonal antibodies and small molecule drugs, are available for the treatment of gastric and breast cancer. These alternatives can limit the uptake of Herceptin biosimilars in some patient populations.
o Additionally, the development of new targeted therapies and personalized medicine approaches may pose a challenge to the growth of the Herceptin biosimilar market.
Herceptin Biosimilar Market Segmentation
The global herceptin biosimilar market segmentation based on the application, end-user and geographical region.
Market by Application
• Breast Cancer
• Colorectal Cancer
• Leukemia
• Lymphoma
• Others
Market by End-User
• Hospital & Clinics
• Oncology Centers
• Others
2. High Costs for Development
o The development of biosimilars is a complex and costly process, requiring significant investment in research and development, clinical trials, and regulatory compliance. These high development costs can be a barrier for smaller companies and new entrants to the market.
o Moreover, the need for rigorous clinical trials to demonstrate biosimilarity and the subsequent regulatory approvals add to the overall cost and time required to bring a biosimilar to market.
3. Significant Side Effects Associated with Herceptin
o Herceptin, like other monoclonal antibody therapies, is associated with certain side effects, including cardiotoxicity, infusion reactions, and pulmonary toxicity. These side effects can limit the use of Herceptin biosimilars, particularly in patients with pre-existing conditions or those who are at higher risk of adverse reactions.
o The management of these side effects requires careful monitoring and may necessitate additional medical interventions, which can increase the overall cost of treatment and impact patient adherence to therapy.
Herceptin Biosimilar Market Players
Currently limited numbers of companies are present in the market. Herceptin was developed by Roche and is still holding the patient in some of the countries. However, the companies like Pfizer, TEVA and others are continuously trying to develop the new generic drugs so as to enter in the market. With the expiry of the patent for the drug in 2019, the herceptin biosimilar market will possess a huge growth. Some of the prominent global herceptin biosimilar market companies are Amgen Inc., Biocon Limited, Merck & Co., Inc., Gedeon Richter Plc, Mabion SA, Roche Holding AG, AryoGen Biopharma, Pfizer Inc., Accord Healthcare Ltd, Genor Biopharma Company Ltd, Mylan N.V, and Samsungbioepis Co., Ltd.
Market Opportunities
1. Strategic Acquisitions and Mergers by Major Influencers
o Strategic acquisitions and mergers among major pharmaceutical companies present significant growth opportunities for the Herceptin biosimilar market. These strategic moves can enhance the capabilities of companies in terms of research and development, manufacturing, and distribution, enabling them to bring biosimilars to market more efficiently.
o Collaborations and partnerships also allow companies to share resources and expertise, reducing the financial burden and risk associated with biosimilar development. These strategic alliances can accelerate the commercialization of Herceptin biosimilars and expand their reach in the global market.
2. Increase in Healthcare Spending
o The global increase in healthcare spending, particularly in emerging economies, is expected to drive the demand for Herceptin biosimilars. Governments and healthcare organizations are investing more in healthcare infrastructure, access to medicines, and innovative treatment options to improve patient outcomes.
o As healthcare budgets increase, there is a growing emphasis on cost-effective treatment options. Herceptin biosimilars, with their potential to reduce treatment costs while maintaining therapeutic efficacy, are well-positioned to benefit from this trend.
3. Rising Demand in Emerging Markets
o Emerging markets, particularly in Asia-Pacific and Latin America, present lucrative opportunities for the Herceptin biosimilar market. These regions have a high prevalence of gastric and breast cancer, and the demand for affordable treatment options is increasing.
o The improving healthcare infrastructure, rising awareness about cancer treatment, and supportive government policies in these regions are expected to drive the adoption of Herceptin biosimilars. Companies that can effectively navigate the regulatory landscape and establish strong distribution networks in these markets are likely to gain a competitive edge.
Herceptin Biosimilar Market Table of Content:
CHAPTER 1. Industry Overview of Herceptin Biosimilar Market
CHAPTER 2. Research Approach
CHAPTER 3. Market Dynamics And Competition Analysis
CHAPTER 4. Herceptin Biosimilar Market Revenue By Application
CHAPTER 5. Herceptin Biosimilar Market Revenue By End-User
CHAPTER 6. North America Herceptin Biosimilar Market By Country
CHAPTER 7. Europe Herceptin Biosimilar Market By Country
CHAPTER 8. Asia-Pacific Herceptin Biosimilar Market By Country
CHAPTER 9. Latin America Herceptin Biosimilar Market By Country
CHAPTER 10. Middle East & Africa Herceptin Biosimilar Market By Country
CHAPTER 11. Player Analysis Of Herceptin Biosimilar Market
CHAPTER 12. Company Profile
Conclusion
The global Herceptin biosimilar market is poised for substantial growth over the forecast period, driven by factors such as the rising prevalence of gastric and breast cancer, strong product pipelines, advancements in healthcare, and endorsements from key companies. While the market faces challenges from alternative therapies, high development costs, and side effects, strategic acquisitions, increased healthcare spending, and opportunities in emerging markets present significant growth prospects. Companies that can effectively address these challenges and capitalize on the opportunities are expected to thrive in the competitive landscape of the Herceptin biosimilar market.
Ask Query Here: Richard@acumenresearchandconsulting.com or sales@acumenresearchandconsulting.com
To Purchase this Premium Report@ https://www.acumenresearchandconsulting.com/buy-now/0/886
Browse for more Related Reports
https://www.globenewswire.com/news-release/2022/08/22/2502270/0/en/Herceptin-Biosimilar-Market-Size-is-expected-to-be-worth-USD-11-287-Million-by-2030-at-a-CAGR-of-23-2-Owing-to-Rising-Prevalence-and-Incidence-of-Gastric-and-Breast-Cancer.html
201, Vaidehi-Saaket, Baner - Pashan Link Rd, Pashan, Pune, Maharashtra 411021
Acumen Research and Consulting (ARC) is a global provider of market intelligence and consulting services to information technology, investment, telecommunication, manufacturing, and consumer technology markets. ARC helps investment communities, IT professionals, and business executives to make fact based decisions on technology purchases and develop firm growth strategies to sustain market competition. With the team size of 100+ Analysts and collective industry experience of more than 200 years, Acumen Research and Consulting assures to deliver a combination of industry knowledge along with global and country level expertise.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Herceptin Biosimilar Market Accelerates 23.2% CAGR Forecast (2022-2030) here
News-ID: 3557872 • Views: …
More Releases from Acumen Research and Consulting
Direct Drive Wind Turbine Market to Reach USD 95.02 Billion by 2035 | Acumen Res …
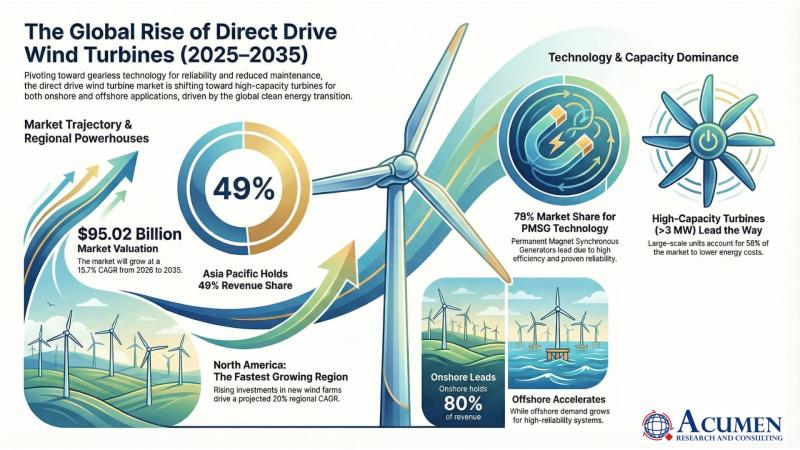
Direct Drive Wind Turbine Market to Reach USD 95.02 Billion by 2035, Driven by Global Renewable Expansion and Offshore Innovation | Acumen Research and Consulting
The Direct Drive Wind Turbine Market is witnessing unprecedented growth momentum as the global renewable energy transition accelerates. According to a new report by Acumen Research and Consulting, the global Direct Drive Wind Turbine Market size is projected to grow from USD 21.91 billion in 2025…
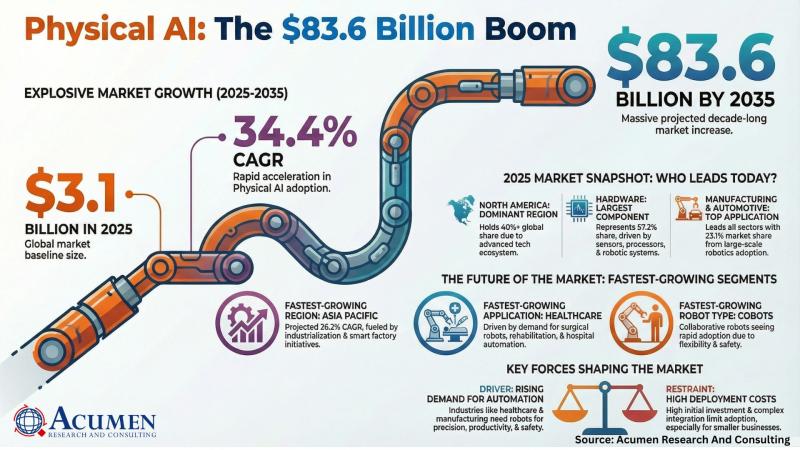
Physical AI Market Set to Surge to USD 83,642.5 Million by 2035 - Groundbreaking …
Global Physical AI Market Report 2026-2035: Robust Growth, Transformational Trends, and Unmatched Opportunities
The Physical AI Market is on the brink of remarkable expansion with groundbreaking advancements in artificial intelligence systems that interact intelligently with the physical world. According to a new market study by Acumen Research and Consulting, the global physical AI market is projected to grow from USD 3,137.5 Million in 2025 to USD 83,642.5 Million by 2035, exhibiting…
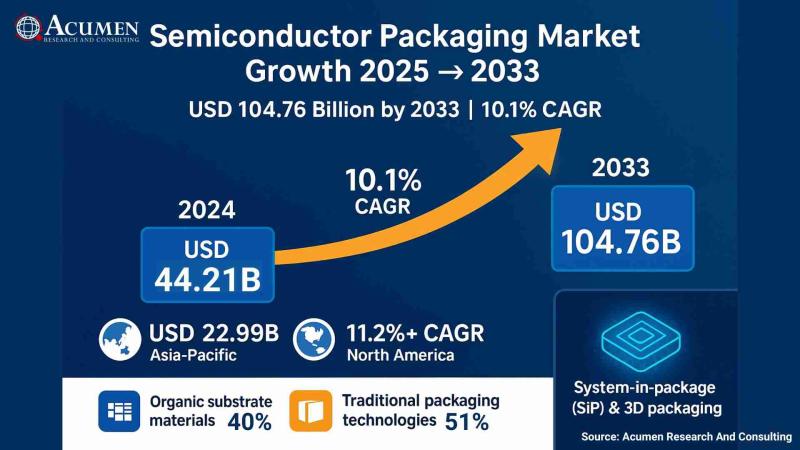
Semiconductor Packaging Market to Double from USD 44.21 Billion in 2024 to USD 1 …
Acumen Research And Consulting announces the release of its latest industry report highlighting the robust growth of the Semiconductor Packaging Market. The report reveals that the global market, valued at USD 44.21 billion in 2024, is projected to reach USD 104.76 billion by 2033, expanding at a steady Compound Annual Growth Rate (CAGR) of 10.1% between 2025 and 2033. This rapid growth underscores the increasing importance of packaging innovations in…
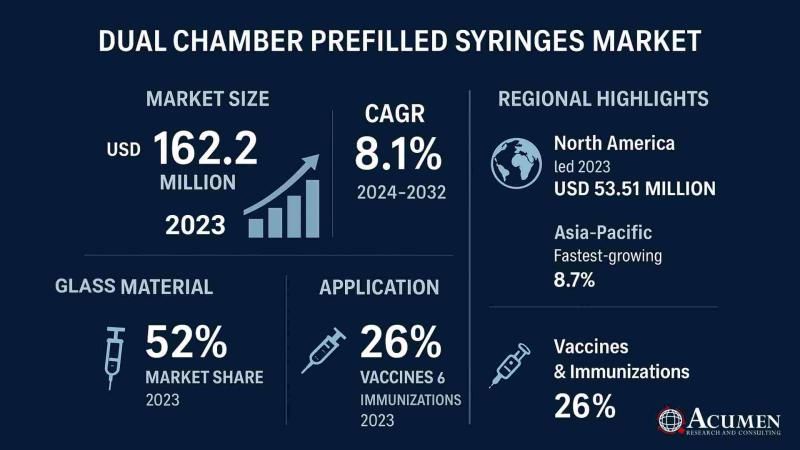
Global Dual Chamber Prefilled Syringes Market to Reach USD 323.7 Million by 2032 …
According to the latest report by Acumen Research and Consulting, the global Dual Chamber Prefilled Syringes Market is witnessing rapid expansion, driven by rising adoption of advanced drug delivery systems, increasing demand for biologics, and the growing emphasis on patient safety and convenience.
The Dual Chamber Prefilled Syringes Market Size was valued at USD 162.2 million in 2023 and is projected to reach USD 323.7 million by 2032, growing at a…
More Releases for Herceptin
Herceptin Biosimilars Market: An Overview
Herceptin (trastuzumab) is a widely used monoclonal antibody that has been pivotal in the treatment of HER2-positive breast cancer. The introduction of biosimilars for Herceptin has revolutionized the pharmaceutical landscape, offering a cost-effective alternative to the original biologic. Biosimilars are highly similar to their reference products in terms of safety, efficacy, and quality, but they are more affordable. As healthcare costs continue to rise globally, biosimilars like Herceptin are becoming…
Herceptin Biosimilar Market Size, Share | Growth - 2030
Exclusive Report by Ameco Research: Herceptin Biosimilar Market Size Projected to Reach USD 11,280 Million by 2030, Growing at 23% CAGR
Ameco Research is proud to announce the launch of its latest market research report, Herceptin Biosimilar Market. This comprehensive report provides in-depth analysis and insights into the current market trends and future projections in the Industry/Market Segment. Ameco Research has been at the forefront of providing quality market research…
Herceptin Biosimilar Market | Industry Trends and Forecast 2022-2030
Acumen Research and Consulting has announced the addition of the "Herceptin Biosimilar Market" report to their offering.
The Herceptin Biosimilar Market Report 2030 is an in depth study analyzing the current state of the Herceptin Biosimilar Market. It provides brief overview of the market focusing on definitions, market segmentation, end-use applications and industry chain analysis. The study on Herceptin Biosimilar Market provides analysis of global market covering the industry trends, recent…
Herceptin Biosimilar Market to Discern Steadfast Expansion During 2029
Global Herceptin Biosimilar Market: Overview
Growing number of people living with gastric cancers and metastatic (spread) breast cancer is expected to drive demand opportunities for enterprises working in the global herceptin biosimilar market. Herceptin refers to a monoclonal antibody mainly utilized in the treatment of critical cancers like gastric cancers and metastatic (spread) breast cancer.
An upcoming research report on the global herceptin biosimilar market provides a complete analysis of this market.…
United States Herceptin Biosimilar Market 2017
In this report, the United States Herceptin Biosimilar Market 2017 is valued at USD XX million in 2016 and is expected to reach USD XX million by the end of 2022, growing at a CAGR of XX% between 2016 and 2022.
Geographically, this report splits the United States market into seven regions:
The West
Southwest
The Middle Atlantic
New England
The South
The Midwest
with sales (volume), revenue (value), market share and growth rate of Herceptin Biosimilar in…
Herceptin (Trastuzumab) Biosimilar Clinical Trial Insight
“Herceptin (Trastuzumab) Biosimilar Clinical Trial Insight” report by PNS Pharma gives comprehensive clinical insight on 37 biosimilar version of Herceptin drug in clinical pipeline. Currently there are 4 biosimilars in Phase-III trials and are expected to be commercially available in next 5-8 years. Currently 3 biosimilar version of Herceptin are commercially available in India and Iran for the treatment of Breast cancer. The patent on Herceptin expired in 2014.
Trastuzumab or…