Press release
Investigational New Drug CDMO Market Powering Drug Discovery Navigating the Early Stages How IND CDMOs Partner with Pharma to Develop New Drugs
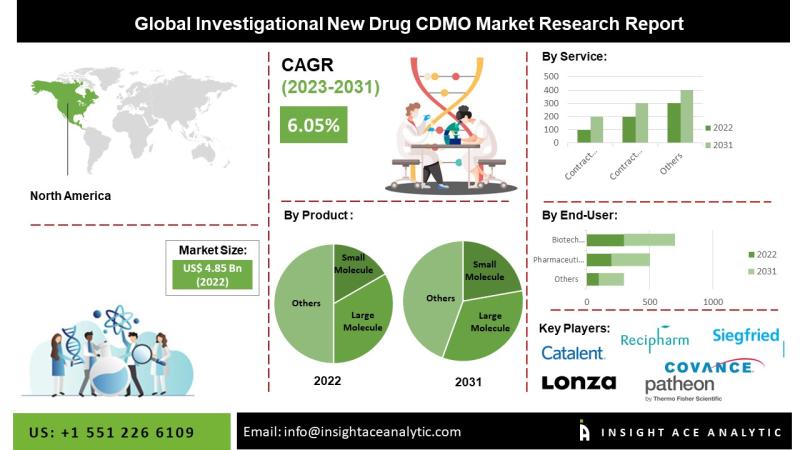
Global Investigational New Drug CDMO Market worth $7.47 billion by 2030 - Exclusive Report by InsightAce AnalyticInsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Investigational New Drug CDMO Market By Product (Small Molecule, Large Molecule), Contract Development (Small Molecule (Bioanalysis and DMPK studies, Toxicology Testing, Pathology and safety pharmacology studies, Drug substance synthetic route development, Drug substance process development, Form selection crystallization process development, Scale-up of drug substance, Preformulation,
Preclinical formulation selection, First in Man Formulation/Process Development, Analytical method development/validation, Release testing of drug substance and drug product, Work up Purification Steps, Telescoping & Process Refining, Initial Optimization, Formal stability of drug substance and drug product)), Large Molecule (Cell Line development, Process Development (Upstream (Microbial, Mammalian, Others), Downstream (MABs,
Recombinant proteins, Others)), Contract Manufacturing (Small Molecule (Oral Solids, Liquid and Semi-solids, Injectables, Others), Large Molecule (MABs, Recombinant proteins, Others))), End-user (Pharmaceutical Companies, Biotech Companies, Others (Government, Research Institutes, Academic Institutes, Etc.))- Trends, Industry Competition Analysis, Revenue and Forecast To 2030."
Get a free sample copy of the report: https://www.insightaceanalytic.com/request-sample/1367
The global Investigational New Drug CDMO market is estimated to reach over USD 7.47 billion by 2024-2031, exhibiting a CAGR of 5.56% during the forecast period.
The Rise of Contract Manufacturing in Pharma: A Lucrative Partnership
The pharmaceutical industry is increasingly turning to Contract Manufacturing and Development Organizations (CMDOs) for clinical and commercial-stage manufacturing. This trend is driven by several factors:
• Capital-intensive Nature of Pharma: The high costs of building and maintaining manufacturing facilities make partnering with a CMDO a financially attractive option.
• Growing Demand for Biologics and Generics: The rising demand for complex biologics and generic drugs creates additional pressure on in-house manufacturing capabilities.
• Intricate Manufacturing Requirements: Modern pharmaceuticals often require specialized expertise and facilities that CMDOs can provide.
Market Growth Fueled by Outsourcing Trends
The CMDO market is experiencing significant growth due to several positive forces:
• Increased Outsourcing by Pharma Companies: Pharmaceutical companies are recognizing the benefits of outsourcing and are focusing their resources on core competencies like drug discovery.
• Rising R&D Investments: Growing investments in research and development are leading to a larger pipeline of potential drugs, further fueling the demand for CMDO services.
• Stringent Regulatory Requirements: The complex and ever-evolving regulatory landscape for clinical trials necessitates expertise that CMDOs can offer.
The Impact of COVID-19
The global pandemic significantly impacted the overall economy, and its effects are still being felt across various industries. However, the IND-focused segment of the CMDO market actually benefited from the pandemic. Due to travel restrictions and social distancing measures, sponsors increasingly relied on virtual facility tours using innovative technologies like virtual reality and 360° videos to assess CMDO capabilities.
In conclusion, the CMDO market is thriving due to a confluence of factors, including the cost advantages of outsourcing, rising demand for complex drugs, and the increasing focus on R&D. While the pandemic presented challenges, it also accelerated the adoption of virtual technologies for CMDO selection. As the pharmaceutical industry continues to evolve, CMDOs are well-positioned to play an even greater role in drug development and manufacturing.
Market Dynamics:
Drivers-
The need for cutting-edge manufacturing methods that have proven to be very effective in satisfying regulatory standards is the primary driver influencing the expansion of Investigational New Drug CDMO in the pharmaceutical sector. Pharmaceutical companies are anticipated to use outsourcing services more frequently, and R&D spending is anticipated to rise.
Challenges:
Lack of high investments will constrain the market for Investigational New Drug CDMO market growth. Moreover, the lack of awareness among individuals in emerging economies limits the market growth. Governments' underinvestment constrains market expansion in medical infrastructure in developing and developing countries.
Regional Trends:
Due to its large patient population and favourable reimbursement policies, the North American market for Investigational New Drugs CDMO is anticipated to have the largest market share soon. This is related to the rise in pharmaceutical and life sciences companies' R&D spending, which is expected to increase the need for contract manufacturing in the region. Market participants are implementing various strategic initiatives, such as new partnership contracts, collaborations,
mergers, and acquisitions, intending to enhance their manufacturing and service offerings to achieve a competitive edge in this area. Additionally, the Investigational New Drug CDMO market in the Asia Pacific is anticipated to expand quickly. The rapid growth of pharmaceutical enterprises and contract manufacturing businesses in developing countries like China and India will likely force the region to grow significantly. Biotech-related companies are expanding there.
There have also been more renowned opinion leaders and principal investigators (PIs). Recently, a series of reforms with the goals of enhancing drug review procedures, speeding up the creation of novel new medications, and decreasing the time required to revise (IND) and NDA applications were announced in China.
Enquiry Before Buying: https://www.insightaceanalytic.com/enquiry-before-buying/1367
Curious about this latest version of the report? Enquiry Before Buying:
Major market players operating in the Investigational New Drug CDMO market include Lonza, Catalent, Recipharm AB, Siegfried Holding AG, Thermo Fisher Scientific Inc., Covance Inc., Charles River Laboratories, Societal CDMO, Inc., Cambrex Corporation, FUJIFILM Diosynth Biotechnologies, Minakem, Regis Technologies Inc., Samsung Biologics, Shanghai Medicilon Inc., and TaiMed Biologics, IQVIA Holdings Inc., Syneous Health, and other prominent players.
Key developments in the market:
• In Aug 2022, Good Manufacturing Practice (GMP) accreditation has been granted to Charles River Laboratories, International Inc. Charles River's Memphis contract development and manufacturing (CDMO) facility's GMP accreditation supplements an existing GMP licence for Investigational Medicinal Product (IMP) manufacture.
• In July 2022, Lonza proposes to invest $518 million in a new fill-finish production facility in Stein, Switzerland. This project will represent the culmination of Lonza's objective to provide fully integrated CDMO services. This investment improves the company's position as a leading CDMO with an unmatched breadth of services across scales and technologies.
• In June 2021, the purchase of Vigene Biosciences, Inc. by Charles River Laboratories International, Inc. The acquisition increased its current cell and gene therapy contract manufacturing capacity and offered a comprehensive gene-modified cell treatment option in the United States.
• In Aug 2019, Permira acquired Cambrex to accelerate growth in the contract development and manufacturing organisation (CDMO) industry, which is consolidating. The investment by the Permira funds will support the continued growth of Cambrex's integrated services offering by enhancing the company's ability to service its global customer base and broadening its capabilities to provide additional world-class services to support the analysis, development, and manufacturing of drug substances and products, from preclinical through commercial phases.
Market Segments
Global Investigational New Drug CDMO Market, by Product, 2020-2030 (Value US$ Mn)
• Small Molecule
• Large Molecule
Global Investigational New Drug CDMO Market, by Service, 2020-2030 (Value US$ Mn)
• Contract Development
o Small Molecule
Bioanalysis and DMPK studies
Toxicology Testing
Pathology and safety pharmacology studies
Drug substance synthetic route development
Drug substance process development
Form selection crystallization process development
Scale-up of drug substance
Preformulation
Preclinical formulation selection
First in Man Formulation/Process Development
Analytical method development/validation
Release testing of drug substance and drug product
Work up Purification Steps
Telescoping & Process Refining
Initial Optimization
Formal stability of drug substance and drug product
o Large Molecule
Cell Line development
Process Development
• Upstream
o Microbial
o Mammalian
o Others
• Downstream
o MABs
o Recombinant proteins
o Others
• Contract Manufacturing
o Small Molecule
Oral Solids
Liquid and Semi-solids
Injectables
Others
o Large Molecule
MABs
Recombinant proteins
Others
Global Investigational New Drug CDMO Market, by End-Users, 2020-2030 (Value US$ Mn)
• Hospitals & Surgical Centers
• Ambulatory Care Centers
• Research Laboratories & Academic Institutes
Global Investigational New Drug CDMO Market, by Region, 2020-2030 (Value US$ Mn)
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa
North America Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• U.S.
• Canada
Europe Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• Germany
• France
• Italy
• Spain
• Russia
• Rest of Europe
Asia Pacific Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• India
• China
• Japan
• South Korea
• Australia & New Zealand
Latin America Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• Brazil
• Mexico
• Rest of Latin America
Middle East & Africa Investigational New Drug CDMO Market, by Country, 2020-2030 (Value US$ Mn)
• GCC Countries
• South Africa
• Rest of Middle East & Africa
Why should buy this report:
To receive a comprehensive analysis of the prospects for the global Investigational New Drug CDMO market
To receive an industry overview and future trends of the Investigational New Drug CDMO market
To analyze the Investigational New Drug CDMO market drivers and challenges
To get information on the Investigational New Drug CDMO market size (Value US$ Mn) forecast to 2030
Major investments, mergers & acquisitions in the Investigational New Drug CDMO market industry
For More Customization @ https://www.insightaceanalytic.com/customisation/1367
Other Related Reports Published by InsightAce Analytic:
Advanced Therapy Medicinal Products CDMO Market
Nucleic Acid Therapeutics CDMO Market
Peptide CDMO (Pharmaceutical) Market
Contact US:
InsightAce Analytic Pvt. Ltd.
Tel.: +1 718 593 4405
Email: info@insightaceanalytic.com
Site Visit: www.insightaceanalytic.com
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Investigational New Drug CDMO Market Powering Drug Discovery Navigating the Early Stages How IND CDMOs Partner with Pharma to Develop New Drugs here
News-ID: 3545531 • Views: …
More Releases from InsightAce Analytic Pvt.Ltd
AI In The Credit-Scoring Market Smarter Credit Decisions: How AI is Transforming …
AI In The Credit-Scoring Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global AI In The Credit-Scoring Market - (By Component (Software and Service), By Application (Personal Credit Scoring and Corporate Credit Scoring), By Industry Vertical (BFSI (Banking, Financial Services, Insurance), Retail, Healthcare,
Telecommunications, Utilities, and Real Estate)), Trends,…
Surgical Robotics Simulation Market Guiding the Next Generation of Surgeons: Gro …
Surgical Robotics Simulation Market Worth $1,283.6 Mn by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Surgical Robotics Simulation Market Size, Share & Trends Analysis Report By Product Type (Product, Services), By Application (General Surgery, Gynecological Surgery, Urological Surgery, Neurological Surgery (Head and Neck Surgery), Cardiological Surgery, Orthopedic Surgery, Others), By End User (Hospitals, Ambulatory Surgical Centers,…
AI Studio Market Accelerating Innovation: The Benefits of AI Studios for Busines …
Global AI Studio Market Worth $57.89 Bn by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global AI Studio Market- (By Application (Sentiment Analysis, Customer Service Automation, Image Classification & Labelling, Synthetic Data Generation, Predictive Modelling & Forecasting, Automatic Content Generation, and Others), By Offering, By Vertical, By Region, Trends, Industry Competition Analysis, Revenue and Forecast…
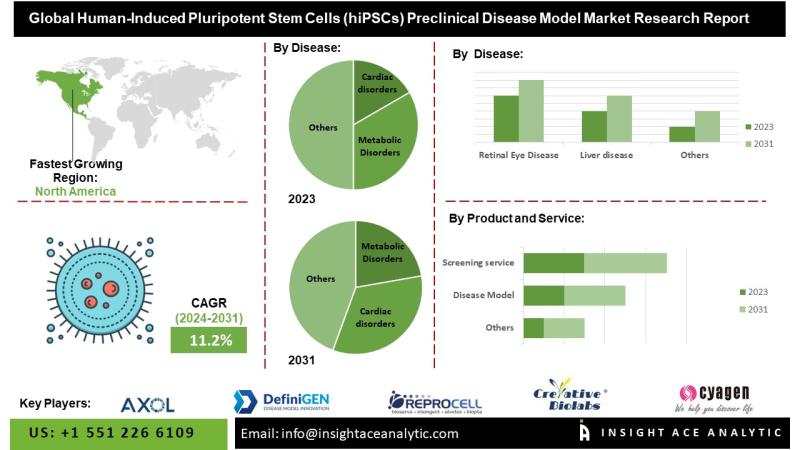
Human-Induced Pluripotent Stem Cells (hiPSCs) Preclinical Disease Model Market G …
Global Human-Induced Pluripotent Stem Cells (hiPSCs) Preclinical Disease Model Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Human-Induced Pluripotent Stem Cells (hiPSCs) Preclinical Disease Model Market- (Disease (Neurological Disorders and Dystrophies, Cardiac disorders, Retinal Eye Disease, Metabolic Disorders, Liver disease, Others), Products and Services (Disease Model,
Reprogramming service, Differentiation…
More Releases for CDMO
FDP CDMO Research: China FDP CDMO market size is projected to reach USD 1.33 bil …
QY Research Inc. (Global Market Report Research Publisher) announces the release of 2025 latest report "Fraud Detection and Prevention (FDP) System- Global Market Share and Ranking, Overall Sales and Demand Forecast 2025-2031". Based on current situation and impact historical analysis (2020-2024) and forecast calculations (2025-2031), this report provides a comprehensive analysis of the global Wire Drawing Dies market, including market size, share, demand, industry development status, and forecasts for the…
Global Cmo And Cdmo Biotechnology Market Size by Application, Type, and Geograph …
According to Market Research Intellect, the global Cmo And Cdmo Biotechnology market under the Internet, Communication and Technology category is expected to register notable growth from 2025 to 2032. Key drivers such as advancing technologies, changing consumer behavior, and evolving market dynamics are poised to shape the trajectory of this market throughout the forecast period.
Biologics and sophisticated medicines are driving the biotechnology industry for Contract Manufacturing Organizations (CMO) and Contract…
Evolving Market Trends In The Inhalation CDMO Industry: Strategic Collaborations …
The Inhalation CDMO Market Report by The Business Research Company delivers a detailed market assessment, covering size projections from 2025 to 2034. This report explores crucial market trends, major drivers and market segmentation by [key segment categories].
What Is the Expected Inhalation CDMO Market Size During the Forecast Period?
In recent times, the inhalation CDMO market has experienced significant growth. The market value is expected to increase from $2.08 billion in 2024…
What's Driving the Inhalation CDMO Market 2025-2034: Rising Respiratory Disorder …
How Is the Chondroplasty Market Projected to Grow, and What Is Its Market Size?
The chondroplasty market has seen strong growth in recent years. It will increase from $13.77 billion in 2024 to $14.68 billion in 2025 at a CAGR of 6.5%. This growth is attributed to the rise in sports-related injuries, patient preference for non-total joint replacement procedures, advances in postoperative care, healthcare provider training, and an increasing incidence of…
Lentiviral Vector (LVV) CDMO Services Market Delivering Cures: The Role of LVV C …
Lentiviral Vector (LVV) CDMO Services Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Lentiviral Vector (LVV) CDMO Services Market - (By Type (IIT Grade, IND Grade, Clinical Trial Grade, Commercial Production Grade), By Application (Biopharmaceutical Company, Academic Scientific Research Institution)), Trends, Industry Competition Analysis, Revenue and Forecast To…
Electronic Chemicals CDMO Market Fueling the Electronics Boom: The Rise of the E …
Electronic Chemicals CDMO Market to Record an Exponential CAGR by 2031 - Exclusive Report by InsightAce Analytic Pvt. Ltd.
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Electronic Chemicals CDMO Market - (By Type (Metals and Pastes, Electronic Specialty Gases, Polymer Compounds, Others), By Application (Battery, Semiconductor, Integrated Circuit, Consumer Electronics, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2031."
According to the latest…