Press release
Clinical Trial Planning and Design Services Market Future Trends and Scope Analysis Report
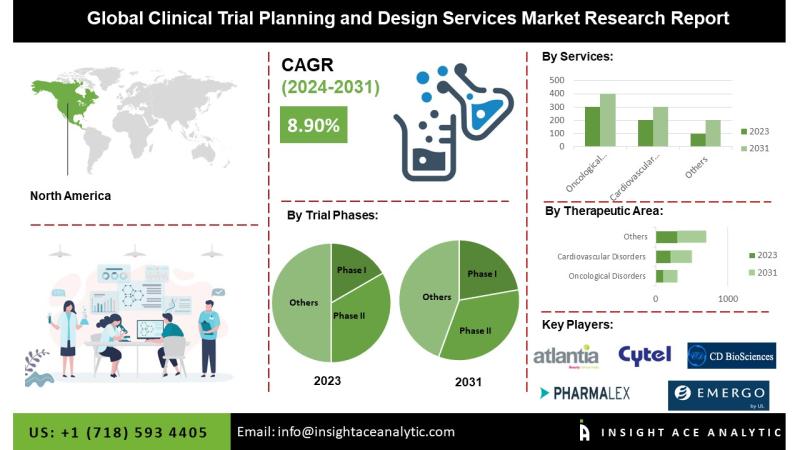
Clinical Trial Planning and Design Services Market Size, Share & Trends Analysis Report By Trial Phase, By Therapeutic Area (Oncological Disorders, Cardiovascular Disorders, Inflammatory Disorders, Neurological Disorders, Other Therapeutic Areas), By Service, By Region, And by Segment Forecasts, 2024-2031.According to a new report by InsightAce Analytic, the "The Clinical Trial Planning and Design Services Market Size is predicted to reach at an 8.90 % CAGR during the forecast period for 2024-2031.
Get A Free Report Brochure: https://www.insightaceanalytic.com/request-sample/1452
Latest Drivers Restraint and Opportunities Market Snapshot:
The following are the primary obstacles to the Clinical Trial Planning and Design Services market expansion:
• Regulatory challenges
• Lengthy approval processes
• Data security and privacy concerns
Key factors influencing the global Clinical Trial Planning and Design Services market are:
• Increasing complexity of clinical trials
• Growing focus on personalized medicine
• Globalization of clinical trials
Future expansion opportunities for the global Clinical Trial Planning and Design Services market include:
• Increased demand for adaptive trial designs
• Focus on patient-centric trials
• Emerging markets
Market Analysis:
The market for clinical trial planning and design services is influenced by various factors, including the complexity of therapeutic interventions, regulatory requirements, technological advancements, and the necessity for efficient trial execution. In an effort to expedite the development and approval of novel treatments, industry participants, such as CROs and speciality service providers, contribute to this market by offering their expertise in a variety of clinical trial planning and design-related areas.
List of Prominent Players in the Clinical Trial Planning and Design Services market:
• Catalent, Inc. QuintilesIMS (now IQVIA)
• ERT (part of LabCorp)
• Wuxi Apptech
• IQVIA Holdings Ltd.
• PPD, Inc. Covance Co., Ltd. (parte a LabCorp)
• Cineos Health Co., Ltd.
• PLC icon
• Parexel International Corporation
• Charles River Laboratories International, Inc.
• Medface Holdings, Inc
Order this Premium Report: https://www.insightaceanalytic.com/buy-report/1452
Recent Developments:
• In 2022, Cytel Inc. broadened its operations to encompass the Asia-Pacific (APAC) region. This will provide biotech and biopharma companies in APAC with access to Cytel's current biometrics and advanced statistical solutions. Cytel currently maintains facilities in Australia, Shanghai, Beijing, and Singapore, with intentions to expand to Tokyo and Seoul in the future. This expansion is the most recent development in Cytel's ongoing endeavor to offer pharmaceutical companies worldwide sophisticated analytics capabilities.
• In 2022, Parexel has disclosed the establishment of a new clinical trial delivery and logistics facility in Suzhou, China. This facility, which is strategically situated, offers local and international biopharmaceutical companies that are conducting clinical trials in the region rapid access to supplies and investigative treatments for distribution to clinical centers and patients worldwide.
Clinical Trial Planning and Design Services Market Dynamics:
Market Drivers: Increasing Complexity Of Clinical Trials
Advancements in medical technology and research are resulting in a growing complexity of clinical trials. In order to guarantee regulatory conformance and efficiency, this complexity necessitates specialized expertise in test planning and design. The demand for clinical trial designs that are adaptable and flexible has been generated by the emphasis on personalized medicine, which customizes medical treatment to the unique characteristics of each individual. Service providers in this market can provide support for research and development that is suitable for personalized medicinal approaches. Pharmaceutical and biotech companies have been pursuing access to a wide range of patient populations and healthcare systems, which has resulted in the globalization of clinical research.
Clinical trial planning services are indispensable for circumventing the obstacles associated with conducting trials across multiple regions. In the pharmaceutical and biotech sectors, it is imperative to comply with regulatory standards. Clinical trial planning and design services assist organizations in navigating the intricate regulatory environment and guarantee that trials remain compliant.
Challenges: High Cost Of Clinical Trials
Clinical investigations are both costly and labor-intensive. Planning and conducting clinical trials can be prohibitively expensive for small biotech companies with restricted financial resources. The time and resources necessary for clinical trials can be augmented by regulatory approval delays. The rapidity with which novel treatments progress through the development pipeline can be impeded by lengthy approval processes. It can be a significant challenge to identify and retain appropriate participants for clinical trials. Process time and success can be impacted by recruitment delays and high attrition rates, necessitating the allocation of additional resources for patient recruitment strategies. The growing dependence on technology and electronic data acquisition in clinical research has led to concerns regarding patient privacy and data security. The planning and design of clinical trials can be complicated by stringent data protection regulations.
Get Specific Chapter/Information From The Report: https://www.insightaceanalytic.com/customisation/1452
North America Is Expected To Grow With The Highest CAGR During The Forecast Period
Several significant trends and drivers have characterized the market for clinical trial planning and design services in North America. It is advisable to consult updated sources for the most current information, as circumstances may have evolved since that time. The United States, in particular, is a significant hub for clinical research and development in North America. A substantial pharmaceutical and biotechnology industry is present in the region, contributing to expanding clinical trial services. The United States, in particular, is a significant contributor to global pharmaceutical R&D expenditure. The demand for clinical trial planning and design services is on the rise due to the increased investment in research and development by pharmaceutical companies. Numerous substantial contract research organizations (CROs) are situated in North America and are crucial in providing clinical trial services. These organizations offer a diverse array of services, such as the planning and design of clinical trials. This region is at the vanguard of the adoption of technological advancements in clinical research. In test plans and design processes, digital tools, electronic data photography (EDC), and other innovations are frequently employed.
Segmentation of Clinical Trial Planning and Design Services Market-
By Therapeutic Area
• Oncological Disorders
• Cardiovascular Disorders
• Inflammatory Disorders
• Neurological Disorders
• Other Therapeutic Areas
By Trial Phases
• Phase I
• Phase II
• Phase III
• Phase IV
By Service
• Statistical Analysis Plan
• eCRF
• Site Identification and Selection
• Medical Writing
• Other Services
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• Southeast Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
Details insights on this market: https://www.insightaceanalytic.com/report/global-clinical-trial-planning-and-design-services-market/1452
Corporate Office :
Office No.5170, 5th Floor Marvel Fuego, Magarpatta Rd, Pune, 411028
loaction icon
Sales Office (U.S.) :
344 Grove St Unit #967 Jersey City, NJ 07302
email iconinfo@insightaceanalytic.com
call icon
North America:
+1 551 226 6109
Asia:
+91 79 72967118
Contact Us:
info@insightaceanalytic.com
InsightAce Analytic Pvt. Ltd.
Visit: www.insightaceanalytic.com
Tel : +1 551 226 6109
Asia: +91 79 72967118
Follow Us on LinkedIn @ bit.ly/2tBXsgS
Follow Us On Facebook @ bit.ly/2H9jnDZ
Twitter: https://twitter.com/InsightaceA
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain a competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets, and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trial Planning and Design Services Market Future Trends and Scope Analysis Report here
News-ID: 3533887 • Views: …
More Releases from Insightace analytic
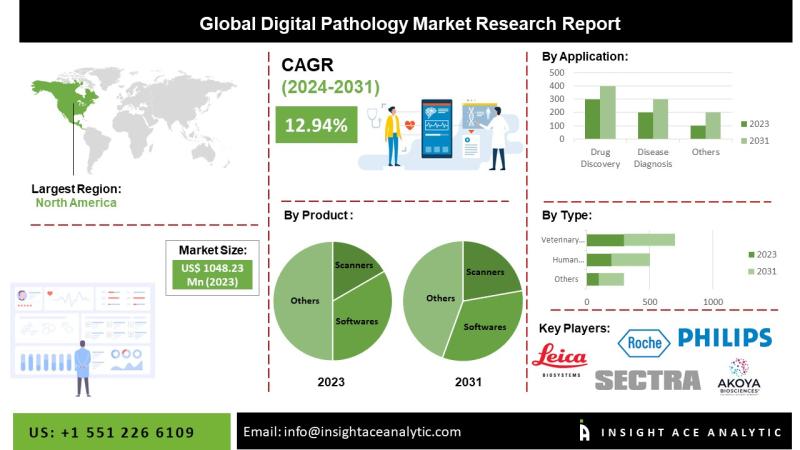
Digital Pathology Market Report- Expansive Coverage on the Profit Sources
InsightAce Analytic announces the release of a market assessment report on the "Global Digital Pathology Market Size, Share & Trends Analysis Report By Product (Artificial Intelligence, Scanner, Software, Storage), Type (Human, Veterinary), Application (Teleconsultation, Training, Disease Diagnosis, Drug Discovery), End User (Pharma, Academia, Hospitals)- Market Outlook And Industry Analysis 2031"
The global digital pathology market is estimated to reach over USD 2731.95 million by 2031, exhibiting a CAGR of 12.91%…
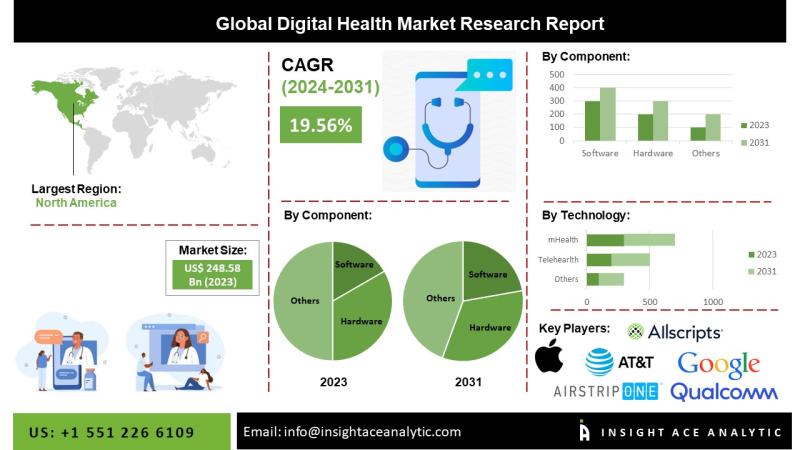
Digital Health Market Future Trends and Scope Analysis Report
Digital Health Market Size, Share & Trends Analysis Report By Technology (Tele-healthcare, mHealth, Healthcare Analytics, Digital Health Systems), By Component (Software, Hardware, Services), By Region, And Segment Forecasts, 2024-2031
"Digital Health Market" in terms of revenue was estimated to be worth $248.58 billion in 2023 and is poised to reach $1,004.03 billion by 2031, growing at a CAGR of 19.56% from 2023 to 2031, according to a new report by…
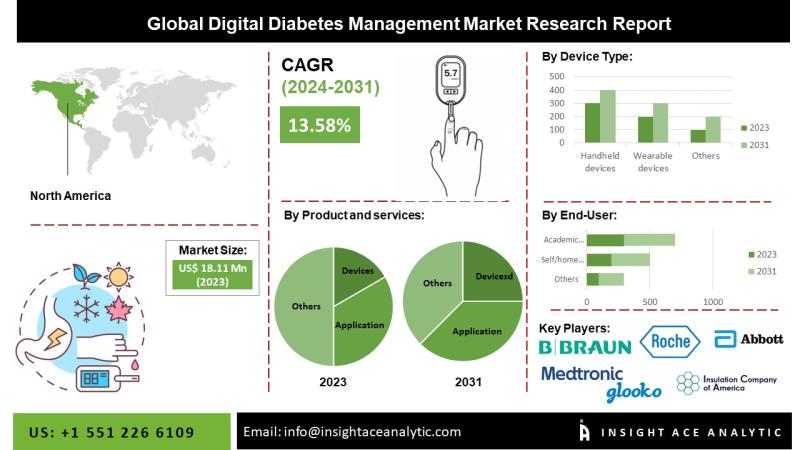
Digital Diabetes Management Market Report on the Untapped Growth Opportunities i …
InsightAce Analytic announces the release of a market assessment report on the "Global Digital Diabetes Management Market - (By Devices (Smart Glucose Meters, Continuous Glucose Monitoring Systems, And Smart Insulin Pens, Smart Insulin Pumps/ Closed-Loop Pumps & Smart Insulin Patches), Application (Diabetes & Blood Glucose Tracking Apps, Obesity & Diet Management Apps), Data Management Software & Platforms And Services), By Device Type (Handheld And Wearable Devices), By End-User(Self/Home Healthcare, Hospitals…
Data Center Liquid Cooling Market Exclusive Trends Analysis with Forecast to 203 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Data Center Liquid Cooling Market Size, Share & Trends Analysis Report By End-User Industry (Financial Services And Insurance (BFSI), Banking, IT And Telecom, Government And Public Sector, Manufacturing, Healthcare, Retail), Data Center (Hyperscale, Colocation, Enterprise) And Solution (Indirect & Direct Cooling)- Market Outlook And Industry Analysis 2031"
The Global Data Center Liquid Cooling Market is estimated…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…