Press release
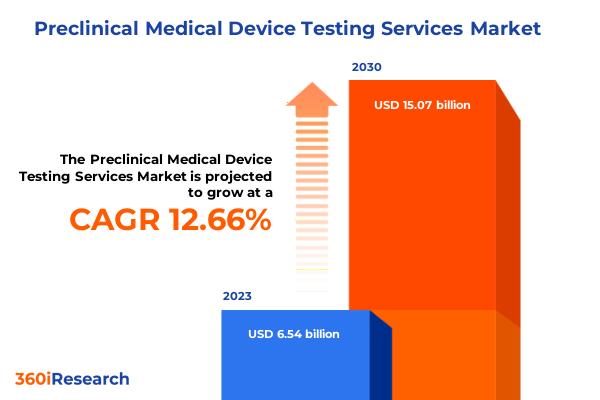
Preclinical Medical Device Testing Services Market worth $15.07 billion by 2030, growing at a CAGR of 12.66% - Exclusive Report by 360iResearch
The "Preclinical Medical Device Testing Services Market by Service (Antimicrobial Activity Testing, Bioburden Determination, Biocompatibility Test), Phase (Antimicrobial Wound Dressings, Medical Coatings) - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/preclinical-medical-device-testing-services?utm_source=openpr&utm_medium=referral&utm_campaign=sample
"Surge in Demand for Preclinical Medical Device Testing Services Driven by Technological Advancements and Regulatory Support"
In response to the growing need for safe and effective medical devices, the preclinical medical device testing services market is experiencing significant growth. This surge is fueled by increasing demand for innovative medical solutions that meet stringent regulatory standards set by bodies such as the FDA and EMA. The expansion of end-user segments such as hospitals, research labs, and pharmaceutical companies, driven by rising healthcare expenditure and advancements in medical technology, further amplifies this growth. Additionally, the high prevalence of chronic diseases necessitating advanced diagnostic and treatment devices has propelled the demand for comprehensive preclinical testing. Strategic partnerships and collaborations within the industry, coupled with technological advancements like imaging techniques, simulation models, and data analytics, have enhanced testing accuracy and efficiency. The economic benefits of early-stage issue identification offered by preclinical services also provide a cost-effective alternative to clinical trials, further solidifying their importance in the development of safe medical devices.
"Market Challenges in Preclinical Medical Device Testing: Key Restraints Hindering Growth"
The preclinical medical device testing services market faces several significant challenges that hinder growth and development. One prominent issue is product recalls due to devices failing to meet safety and performance standards, which not only causes financial losses but also tarnishes brand reputation and stakeholder trust. Additionally, the unavailability of high-quality raw materials can disrupt supply chains, delaying testing processes and escalating costs. The high expense of advanced preclinical testing services poses a barrier for smaller companies, while stringent regulatory standards and lengthy approval processes further obstruct market entry for new testing methodologies. Compatibility and product standardization issues across different regions exacerbate inconsistencies in testing procedures and results. Moreover, the rise of alternative technologies, such as in silico modeling, threatens the demand for traditional preclinical testing services by offering faster and more cost-effective solutions. Addressing these challenges is essential for stakeholders to navigate the complex landscape and foster a more efficient and reliable preclinical testing environment.
"Press Release Title: Transformative Trends in Preclinical Medical Device Testing: Technological Innovations and Market Expansion"
The landscape of preclinical medical device testing services is being revolutionized by continuous technological advancements and substantial investment in research and development (R&D). Cutting-edge tools, such as advanced imaging and simulation software, are enhancing the precision and efficiency of testing, reducing time-to-market and improving the safety and quality of devices. Increased R&D funding from both public and private sectors is driving the development of innovative testing methodologies, ensuring thorough vetting of new medical devices before clinical trials. The expansion into new market segments, including wearable and remote monitoring devices, necessitates specialized testing protocols tailored to diverse conditions, thereby increasing device reliability and readiness. Collaborative initiatives among industry players, academic institutions, and research organizations are fostering the development of advanced testing methods and accelerating regulatory approvals. Government policies that support medical innovation and provide clear guidelines and funding are critical in bolstering this sector. Furthermore, advancements in production technologies, such as 3D printing and automation, allow for the creation of complex prototypes that undergo rigorous preclinical testing, ensuring their effectiveness before clinical trials. Finally, the vibrant start-up ecosystem within the medical device industry drives demand for specialized preclinical testing services, contributing to market diversity and the introduction of novel technologies requiring comprehensive validation.
"Navigating Complex Challenges in Preclinical Medical Device Testing: Counterfeits, Skilled Workforce Shortages, and Environmental Concerns"
Preclinical medical device testing services encounter multifaceted challenges in product development and manufacturing, necessitating intricate design, rigorous testing protocols, and adherence to stringent regulatory standards, which often extend development time and increase costs. The presence of counterfeit medical devices in the market further exacerbates risks to patient safety, potentially resulting in erroneous test results and undermining the credibility of legitimate manufacturers and testing services. The industry also faces a critical shortage of skilled professionals, including engineers, biologists, and regulatory experts, crucial for the successful development, validation, and approval of new devices. This scarcity can hinder innovation, delay project timelines, and inflate operational costs. Additionally, integrating new devices into existing healthcare systems is complex, requiring compatibility with current technologies and extensive training for healthcare providers. Environmental impacts of manufacturing and disposing of medical devices add another layer of concern, with significant waste production and hazardous materials management posing substantial challenges and costs to ensure responsible practices.
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/preclinical-medical-device-testing-services?utm_source=openpr&utm_medium=referral&utm_campaign=inquire
Market Segmentation & Coverage:
This research report categorizes the Preclinical Medical Device Testing Services Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Service, market is studied across Antimicrobial Activity Testing, Bioburden Determination, Biocompatibility Test, Chemistry Test, Microbiology Test & Sterility, Pyrogen & Endotoxin Testing, and Sterility Test & Validation.
Based on Phase, market is studied across Antimicrobial Wound Dressings and Medical Coatings.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom.
Key Company Profiles:
The report delves into recent significant developments in the Preclinical Medical Device Testing Services Market, highlighting leading vendors and their innovative profiles. These include Bureau Veritas S.A., Charles River Laboratories, Inc., Eurofins Scientific (Ireland) Limited, Intertek Group PLC, Medistri SA, Nelson Labs NV, PaxeraHealth LLC, Pharmacology Discovery Services Taiwan, Ltd., SGS Société Générale de Surveillance SA, Sipra Labs Limited, Sterigenics International LLC, TÜV Rheinland AG, UL LLC, Viroclinics Biosciences, and WuXi AppTec Co., Ltd..
Introducing Query Query: Revolutionizing Market Intelligence with AI-Powered Insights for the Preclinical Medical Device Testing Services Market
We proudly unveil Query Query, a cutting-edge AI product designed to transform how businesses interact with the Preclinical Medical Device Testing Services Market. Query Query stands out as your premier market intelligence partner, delivering unparalleled insights with the power of artificial intelligence. Whether deciphering market trends or offering actionable intelligence, Query Query is engineered to provide precise, relevant answers to your most critical business questions. This revolutionary tool is more than just an information source; it's a strategic asset that empowers your decision-making with up-to-the-minute data, ensuring you stay ahead in the fiercely competitive Preclinical Medical Device Testing Services Market. Embrace the future of market analysis with Query Query, where informed decisions lead to remarkable growth.
Ask Question to Query Query @ https://www.360iresearch.com/library/intelligence/preclinical-medical-device-testing-services?utm_source=openpr&utm_medium=referral&utm_campaign=query
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Preclinical Medical Device Testing Services Market, by Service
7. Preclinical Medical Device Testing Services Market, by Phase
8. Americas Preclinical Medical Device Testing Services Market
9. Asia-Pacific Preclinical Medical Device Testing Services Market
10. Europe, Middle East & Africa Preclinical Medical Device Testing Services Market
11. Competitive Landscape
12. Competitive Portfolio
Read More @ https://www.360iresearch.com/library/intelligence/preclinical-medical-device-testing-services?utm_source=openpr&utm_medium=referral&utm_campaign=analyst
Contact 360iResearch
Mr. Ketan Rohom
Sales & Marketing,
Office No. 519, Nyati Empress,
Opposite Phoenix Market City,
Vimannagar, Pune, Maharashtra,
India - 411014.
sales@360iresearch.com
+1-530-264-8485
+91-922-607-7550
About 360iResearch
360iResearch is a market research and business consulting company headquartered in India, with clients and focus markets spanning the globe.
We are a dynamic, nimble company that believes in carving ambitious, purposeful goals and achieving them with the backing of our greatest asset - our people.
Quick on our feet, we have our ear to the ground when it comes to market intelligence and volatility. Our market intelligence is diligent, real-time and tailored to your needs, and arms you with all the insight that empowers strategic decision-making.
Our clientele encompasses about 80% of the Fortune Global 500, and leading consulting and research companies and academic institutions that rely on our expertise in compiling data in niche markets. Our meta-insights are intelligent, impactful and infinite, and translate into actionable data that support your quest for enhanced profitability, tapping into niche markets, and exploring new revenue opportunities.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Preclinical Medical Device Testing Services Market worth $15.07 billion by 2030, growing at a CAGR of 12.66% - Exclusive Report by 360iResearch here
News-ID: 3532769 • Views: …
More Releases from 360iResearch

Rising Incidence of Human Metapneumovirus in Vulnerable Populations Boosts Globa …
In recent years, the silent ascent of human metapneumovirus (HMPV) infections has begun to capture significant attention within the realm of infectious diseases. As we advance in medical science, unraveling complexities of age-old pathogens like common influenza or emerging illnesses like COVID-19, a critical discourse has been emerging around HMPV. Particularly, there seems to be a burgeoning acknowledgment of its growing impact on vulnerable global populations, propelling an increased demand…

The Meat Alternatives Market size was estimated at USD 9.39 billion in 2023 and …
From Appetite to Advocacy: The Rising Demand for Meat Alternatives
In recent years, the global food industry has been undergoing a remarkable transformation, driven primarily by an increasing consumer demand for healthier, sustainable, and ethically sourced food products. This seismic shift has brought traditional meat alternatives and high-protein plant-based foods into the spotlight. As the world becomes more conscious of the implications of meat consumption on health and the environment, the…

The Mobility-as-a-Service Market size was estimated at USD 264.80 billion in 202 …
Unpacking the Surge in Investments and Collaborations to Bolster Mobility-as-a-Service
In recent years, as urban landscapes continually evolve, a transformative shift known as Mobility-as-a-Service (MaaS) has reshaped the way we perceive transportation. Marked by the integration of various forms of transport services into a single accessible on-demand mobility solution, MaaS is rapidly gaining traction across global cities. As an emerging paradigm, it's not just shaping the future of travel but also…

The Data Center Services Market size was estimated at USD 56.65 billion in 2023 …
Smart City Revolutions: Why Data Center Colocation is the Future Backbone
In the era of digital transformation, urban landscapes across the globe are undergoing a seismic shift toward becoming "smart cities." The concept of a smart city revolves around using digital technology, IoT (Internet of Things), AI, and data analytics at an unprecedented scale to improve urban infrastructure, manage resources efficiently, and enhance the quality of life for citizens. A vital…
More Releases for Test
Key Differences Between Megger Test, Tan Delta Test, and Hi-Pot Test for Electri …
Electrical insulation plays a critical role in ensuring the safety and efficiency of electrical systems. To assess the condition of insulation and identify potential issues, three common tests are used: the Megger test, Tan Delta test, and Hi-Pot test. Each test serves a unique purpose and provides valuable insights into the state of electrical insulation. Here's a closer look at the differences between these three essential tests.
Megger Test: Insulation Resistance…
Vitamin Test Market: Global Vitamin Test Analysis and Forecast (2023-2029)Vitami …
12.04.2024: Vitamin Test Market Overview
The development of companion diagnostic tools and advances in personalised treatment are driving considerable growth and revolution in the oncology Vitamin Test market. In the era of precision medicine, where healthcare is increasingly customised for individual individuals based on their own genetic and molecular profiles, this market segment is essential. Ongoing innovation and development define the oncology Vitamin Test market. To find particular biomarkers, genetic mutations,…
CAGR 8.1% Homecare Pregnancy Test Kits Market By Type of Test (Urine Test For H …
The Homecare Pregnancy Test Kits market report by Reports and Data provides an extensive overview of the vital elements of the Homecare Pregnancy Test Kits market and factors such as the drivers, restraints, latest trends, supervisory scenario, competitive landscape, technological advancements, and others. Further, it mentions the market shares associated with the market in terms of both value and volume along with the segmentation. Space-age industrial and digitalization tools are…
Home Safety Test Kits Market, Home Safety Test Kits Market Trends, Home Safety T …
“Home Safety Test Kits Market” 2020-2025 Research Report is a professional and in-depth study on the current state of the market. Global Home Safety Test Kits market containing a complete view of the market size, business share, profit estimates, SWOT analysis and the regional landscape of the Industry. The report explains key challenges and future development prospects of the market. The Global Home Safety Test Kits analysis is provided for…
Test Data Management (TDM) Market - test data profiling, test data planning, tes …
The report categorizes the global Test Data Management (TDM) market by top players/brands, region, type, end user, market status, competition landscape, market share, growth rate, future trends, market drivers, opportunities and challenges, sales channels and distributors.
This report studies the global market size of Test Data Management (TDM) in key regions like North America, Europe, Asia Pacific, Central & South America and Middle East & Africa, focuses on the consumption…
Hearing Screening and Diagnostic Devices Market Demands with Major Tests: pure T …
New Market Research Reports Title "Hearing Screening And Diagnostic Devices Market 2018" Has Been Added to Crystal Market Research Report database.
Hearing Screening and Diagnostic Devices - Competitive Insights:
The leading players in the market are Gn Otometrics A/S, Otodynamics, Nashua Hearing Group, Siemens Healthineers, Natus Medical Incorporated, Interacoustics A/S, Neurosoft S.A, Accent Hearing Pty Ltd, MAICO Diagnostics GmbH and IntriCon Corporation. The major players in the market are profiled in detail…