Press release
Fabry Disease Treatment Market: A Catalyst for Innovation with Diverse Treatment Options
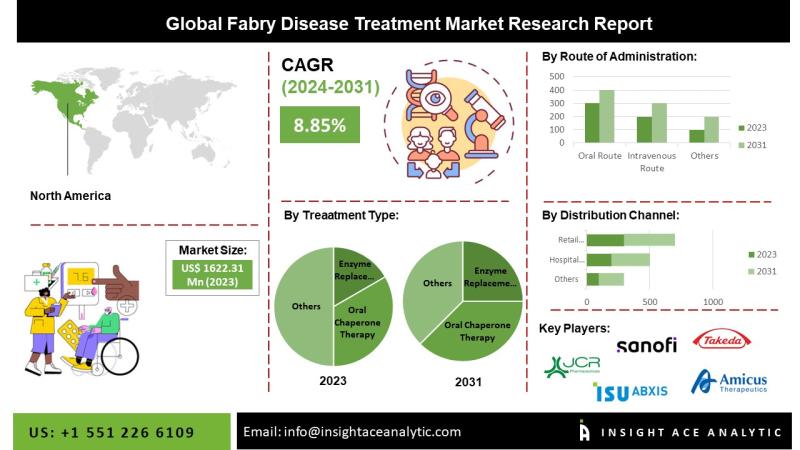
"Fabry Disease Treatment Market" in terms of revenue was estimated to be worth $1,622.31 Mn in 2023 and is poised to reach $3,150.75 Mn by 2031, growing at a CAGR of 8.21% from 2024 to 2031 according to a new report by InsightAce Analytic.Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/1451
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the Fabry disease treatment market are:
• Raise your level of awareness
• Advances in research and development
• Designated orphan drug
The following are the primary obstacles to the fabry disease treatment market's expansion:
• Limited number of patients
• High cost of treatment
• Difficulty in diagnosis
Future expansion opportunities for the global Fabry disease treatment market include:
• Advances in research and development
• Pipeline development
• International expansion
Market Analysis:
The Fabry disease treatment market includes several treatment approaches to manage the symptoms and underlying causes of Fabry disease. The main treatment for Fabry disease is enzyme replacement therapy. This is associated with the injection of the composite version of alpha-galactose Dadase A, which is deficient in patients with Fabry disease. Sharpon therapy includes the use of a small molecule called a Chaperon to stabilize and improve the activity of defective enzymes. Matrix-reducing therapy aims to reduce the production of matrix (Gb3), which accumulates in Fabry disease. Ongoing clinical trials are essential to test the safety and effectiveness of new treatments for Fabry disease.
List of Prominent Players in the Fabry Disease Treatment Market:
• Sanofi Genzyme
• Share
• Amicus therapeutics
• Protalix biotherapeutics
• Idorsia pharmaceuticals
• Migal Galilee
• Greenovation biotech gmbh
• Chiesi group
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1451
Recent Developments:
• In May 2023, Europe granted Chiesi Farmaceutici and Protalix BioTherapeutics marketing authorization for PRX-102 (pegungalsidase alfa) for the treatment of Fabry disease in Europe. This approval will help expand treatment options for patients with Fabry disease in the region.
• In September 2022, the FDA granted Orphan Drug Designation (ODD) to AL01211 for the treatment of Fabry disease, developed by AceLink Therapeutics. This particular treatment, a glucosylceramide synthase inhibitor (GCS inhibitor), is unique because it is an oral medication that fills a significant need compared to other treatments.
• In August 2018, PerkinElmer received approval from the U.S. Food and Drug Administration (FDA) to sell the NeoLSD MSMS kit commercially. This innovative tool can detect approximately six lysosomal storage disorders in newborns, including Fabry disease, and can be easily diagnosed using a blood sample.
Fabry Disease Treatment Market Dynamics:
Market Drivers: Increasing Awareness among Medical Professionals
As awareness among medical professionals, patients and the general public about rare diseases such as Fabry disease increases, the demand for effective treatments is expected to increase. Continued research to understand Fabry's disease and develop new treatment options can fuel market growth. Innovations in gene therapy, enzyme replacement therapy, and other therapeutic approaches may expand the therapeutic landscape. Fabry disease is classified as a rare or orphan disease, and many regulatory agencies offer incentives to develop treatments for such diseases. These incentives may include market exclusivity, tax breaks, and exemptions from regulatory fees to encourage pharmaceutical companies to invest in developing treatments for Fabry disease. Collaboration between pharmaceutical companies, academic institutions, and research institutions can accelerate the development of new treatments. These partnerships leverage resources, expertise, and funding to bring new treatments to market.
Challenges: Limited Patient Population
Fabry disease is a rare genetic disease with a relatively small number of patients. The limited number of patients with Fabry disease can present challenges to pharmaceutical companies in terms of the amount of investment required for research, development, and marketing. Developing and manufacturing treatments for rare diseases can be expensive. As a result, the cost of treating Fabry disease can be high. This can hinder patient access and reimbursement, particularly in regions with cost-sensitive health systems. Fabry disease can be difficult to diagnose because of diverse and nonspecific symptoms, and symptoms can overlap with other diseases. Delays or inaccuracies in diagnosis can prevent the timely initiation of treatment and affect market growth. Complying with the regulatory requirements for approval of new treatments for Fabry disease can be a complex and time-consuming process. Navigating the regulatory pathway, obtaining orphan drug status, and meeting stringent clinical trial requirements are among the challenges companies may face. The Fabry disease treatment market can face challenges due to competition, especially when there are too many treatment options. Market saturation, where different products offer similar therapeutic effects, can affect market share and pricing strategies for individual treatments.
North America Is Expected To Grow With The Highest CAGR During The Forecast Period
North America plays an important role in the Fabry disease treatment market. The region is characterized by an established healthcare infrastructure, a regulatory framework that supports the development of orphan drugs, and a relatively high prevalence of rare diseases. Enzyme replacement therapy is one of the main treatment options for Fabry disease, and several ERTs have been approved in North America. These treatments are designed to address the root cause of Fabry disease by replacing the missing alpha-galactosidase A enzyme. Many rare disease treatments, including Fabry disease, have been granted orphan drug status by North American regulators. This designation encourages pharmaceutical companies and encourages the development of treatments for rare diseases. North America is a center for clinical trials for rare diseases, including Fabry disease. Clinical research and trials are essential to test the safety and effectiveness of new treatments and therapies. Patients in North America often have access to innovative treatments by participating in clinical trials. Patient advocacy groups in North America play an important role in raising awareness of Fabry disease, providing support to patients and their families, and improving access to treatment. These groups often work with researchers, medical professionals, and pharmaceutical companies to improve the understanding and treatment of Fabry disease.
Segmentation of Fabry Disease Treatment Market-
By Type Of Treatment-
• Enzyme replacement therapy (ERT)
• Oral Chaperone Therapy
• Other Treatments
By Route of Administration
• Oral Route
• Intravenous Route
By Distribution Channel
• Hospital Pharmacies
• Retail Pharmacies
• Online Pharmacies
By Region-
North America-
• The US
• Canada
• Mexico
Europe-
• Germany
• The UK
• France
• Italy
• Spain
• Rest of Europe
Asia-Pacific-
• China
• Japan
• India
• South Korea
• South East Asia
• Rest of Asia Pacific
Latin America-
• Brazil
• Argentina
• Rest of Latin America
Middle East & Africa-
• GCC Countries
• South Africa
• Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1451
InsightAce Analytic Pvt. Ltd.
Tel.: +1 551 226 6109
Email: info@insightaceanalytic.com
Site Visit: www.insightaceanalytic.com
Follow Us on LinkedIn @ bit.ly/2tBXsgS
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Fabry Disease Treatment Market: A Catalyst for Innovation with Diverse Treatment Options here
News-ID: 3510651 • Views: …
More Releases from InsightAce Analytic Pvt. Ltd
Sustainable Packaging Market Strategic Industry Overview and Forecast 2026 to 20 …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Sustainable Packaging Market Size, Share & Trends Analysis Report By Material (Paper & Paperboard, Plastic, Metal, Glass), Process (Recycled, Reusable, Degradable), Function (Active, Molded Pulp, Alternate Fiber), Application (Food & Beverage, Healthcare, Others) & Layer (primary, secondary, and tertiary)- Market Outlook And Industry Analysis 2034"
The global Sustainable Packaging Market is estimated to reach over USD…
SCADA Market Insights Highlighting Technological Advancements in Wireless Sensor …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global SCADA Market Size, Share & Trends Analysis Report By Offering (Hardware, Software, Services), Component (Programmable Logic Controller, Remote Terminal Unit, Human-Machine Interface), End User (Process Industries, Discrete Manufacturing, Utilities), Region, Market Outlook And Industry Analysis 2034"
The global SCADA market is estimated to reach over USD 25.0 billion by the year 2034, exhibiting a CAGR of…
Biocomputer Based on Organoids Market Poised for 16.3% CAGR Driven by Brain Orga …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Biocomputer Based on Organoids Market Size, Share & Trends Analysis Report By Organoid Type (Brain Organoids, Other Organoids), Application (Biological Computing, Neuroscience Research, Drug Discovery and Testing, Personalized Medicine, Regenerative Medicine), Technology (Microfluidic Systems Microelectrode Arrays (MEAs), Brain-Machine Interfaces, CRISPR and Gene Editing), End-User (Academic and Research Institutes, Biotechnology and Pharmaceutical Companies, Technology Companies, Contract…
Postbiotic Supplements Market Drivers Include Functional Nutrition and Bioactive …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Postbiotic Supplements Market - (By Type (Bacteria, Yeast), By Form (Soft gels, Capsules/Tablets, Powder/ Granules, Others), By Application (Personal Care and Cosmetics, Food and Beverages, Animal Feed, Pharmaceuticals, Others)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Postbiotic Supplements Market is valued at USD 12.8…
More Releases for Fabry
Key Factor Supporting Global Fabry Disease Treatment Market Development in 2025: …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Fabry Disease Treatment Market Size By 2025?
The valuation of the Fabry disease treatment market has experienced robust expansion recently, projected to advance from $2.09 billion in 2024 up to $2.27 billion by 2025, reflecting an 8.8% compound annual growth rate during this timeframe. This…
U.S. Fabry Disease Market Size Report 2034
On April 28, 2025, Exactitude Consultancy., Ltd. released a research report titled "U.S. Fabry Disease Market". This report covers the global U.S. Fabry Disease market sales, sales volume, price, market share, ranking of major companies, etc., and provides a detailed analysis by region, country, product type, and application. It also forecasts the market size of automotive kick sensors based on market patterns from 2020 to 2034 and future market trends.…
Top Factor Driving Fabry Disease Treatment Market Growth in 2025: Impact of Incr …
How Are the key drivers contributing to the expansion of the fabry disease treatment market?
The rising prevalence of renal diseases is expected to drive the growth of the Fabry disease treatment market. Renal diseases are becoming more prevalent due to genetic factors, lifestyle choices, and environmental influences. Fabry disease, which causes kidney dysfunction, is increasing and requires timely intervention. According to the Australian Bureau of Statistics, kidney disease affected 246,200…
Fabry Disease Market Trends Analysis 2030
Fabry disease is a rare X-linked lysosomal storage disorder. This patient has a deficiency in the enzyme alpha galactosidase, which progresses to organ failure. The development of fabry illnesses is mostly caused by abnormal accumulation of a certain fatty substance known as globotriaosylceramide. This aberrant buildup can be detected in the skin, eyes, heart, kidney, brain, gastrointestinal system, and central nervous system, among other body parts.
Galactosidase Alpha (GLA) is a…
Fabry Disease - Pipeline Review, H1 2017
ReportsWorldwide has announced the addition of a new report title Fabry Disease - Pipeline Review, H1 2017 to its growing collection of premium market research reports.
Global Markets Direct's latest Pharmaceutical and Healthcare disease pipeline guide Fabry Disease - Pipeline Review, H1 2017, provides an overview of the Fabry Disease (Genetic Disorders) pipeline landscape.
Fabry disease is an inherited disorder. Fabry disease results from abnormal deposits of a particular fatty substance (called…
Fabry Disease Market Intelligence Report Offers Growth Prospects
Fabry diseaseis also known as Anderson-Fabry disease and alpha-galactosidase A deficiency. It is a rare genetic disorder of lipid metabolism resulting from the deficient activity of the alpha-galactosidase A (a-Gal A) enzyme. The deficiency of the enzyme is caused by the alterations in the genes that instructs the cells to make alpha-galactosidase A (a-Gal A) enzyme. Fabry disease is known to cause variety of systemic symptoms and complications, one of…