Press release
Lewy Body Disease Pipeline Assessment, 2024 Updates | In-depth Insights Into the Clinical Trials, Emerging Drugs, Latest Approvals, Treatment Outlook, Companies | Annovis Bio,CervoMed,Vaxxinity
As per DelveInsight's assessment, globally, about 15+ key pharma and biotech companies are working on 15+ pipeline drugs in the Lewy Body Disease therapeutics landscape based on different Routes of Administration (ROA), Mechanism of Action (MOA), and molecule types. Several of the therapies are in the advanced stages of clinical development and are expected to launch in the coming years." [https://www.delveinsight.com/report-store/lewy-body-disease-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]" report by DelveInsight outlines a comprehensive assessment of the present clinical/non-clinical development activities and growth prospects across the Lewy Body Disease Market.
The Lewy Body Disease Pipeline report embraces in-depth commercial and clinical assessment of the pipeline products from the pre-clinical developmental phase to the marketed phase. The report also covers a detailed description of the drug, including the mechanism of action of the drug, clinical studies, NDA approvals (if any), and product development activities comprising the technology, collaborations, mergers, acquisition, funding, designations, and other product-related details.
[https://www.delveinsight.com/report-store/lewy-body-disease-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
The report provides insights into:
*
The report provides detailed insights into the emerging therapies for the treatment of Lewy Body Disease and the aggregate therapies developed by major pharma companies.
*
It accesses the different therapeutic candidates segmented into early-stage, mid-stage, and late-stage of clinical development.
*
It outlines the key companies involved in targeted therapeutics development with respective active and inactive (dormant or discontinued) projects.
*
The report evaluates the drugs that are under development based on the stage of development, route of administration, target receptor, monotherapy or combination therapy, a different mechanism of action, and molecular type.
*
It navigates the major collaborations (company-company collaborations and company-academia collaborations), licensing agreements, financing details, data presentation by the pharma giants, and regulatory approval in the Lewy Body Disease market.
The report is built using data and information traced from the researcher's proprietary databases, company/university websites, clinical trial registries, conferences, SEC filings, investor presentations, and featured press releases from company/university websites and industry-specific third-party sources, etc.
Analysis of Emerging Therapies by Phases
The report covers the emerging products under different phases of clinical development -
*
Late stage products (Phase III)
*
Mid-stage products (Phase II)
*
Early-stage product (Phase I)
*
Pre-clinical and Discovery stage candidates
*
Discontinued & Inactive candidates
Route of Administration
Lewy Body Disease pipeline report provides the therapeutic assessment of the pipeline drugs by the Route of Administration. Products have been categorized under various ROAs, such as
*
Oral
*
Parenteral
*
Intravenous
*
Subcutaneous
*
Topical
Molecule Type
Products have been categorized under various Molecule types, such as
*
Monoclonal Antibody
*
Peptides
*
Polymer
*
Small molecule
*
Gene therapy
Learn How the Ongoing Clinical & Commercial Activities will Affect the Lewy Body Disease Therapeutic Segment @ [https://www.delveinsight.com/sample-request/lewy-body-disease-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Lewy Body Disease Therapeutics Landscape
There are approx. 15+ key companies are developing therapies for Lewy Body Disease. Currently, Eli Lilly and Company is leading the therapeutics market with its Lewy Body Disease drug candidates in the most advanced stage of clinical development.
The Leading Companies in the Lewy Body Disease Therapeutics Market Include:
*
Eisai Inc.
*
EIP Pharma
*
KeifeRx
*
Generian Pharmaceuticals, Inc.
*
Aptinyx
*
Eli Lilly and Company
*
Sun Pharma Advanced Research Company
*
CuraSen Therapeutics
*
NeuroActiva
*
Neurimmune Therapeutics
*
Voyager Therapeutics
*
Yumanity Therapeutics
*
Immungenetics
*
ProMIS Neurosciences
*
NLS Pharmaceutics Ltd
*
Inhibikase Therapeutics
And many others
Lewy Body Disease Drugs Covered in the Report Include:
*
Irsenontrine (E 2027): Eisai Inc
*
Neflamapimod: EIP Pharma
*
Mevidalen: Eli Lilly and Company
And many more
Request the Sample PDF to Get a Better Understanding of the Emerging Drugs and Key Companies @ [https://www.delveinsight.com/sample-request/lewy-body-disease-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Table of Contents
1. Report Introduction
2. Executive Summary
3. Lewy Body Disease Current Treatment Patterns
4. Lewy Body Disease - DelveInsight's Analytical Perspective
5. Therapeutic Assessment
6. Lewy Body Disease Late Stage Products (Phase-III)
7. Lewy Body Disease Mid-Stage Products (Phase-II)
8. Lewy Body Disease Early Stage Products (Phase-I)
9. Pre-clinical Products and Discovery Stage Products
10. Inactive Products
11. Dormant Products
12. Lewy Body Disease Discontinued Products
13. Lewy Body Disease Product Profiles
14. Key Companies in the Lewy Body Disease Market
15. Key Products in the Lewy Body Disease Therapeutics Segment
16. Dormant and Discontinued Products
17. Lewy Body Disease Unmet Needs
18. Lewy Body Disease Future Perspectives
19. Lewy Body Disease Analyst Review
20. Appendix
21. Report Methodology
*The Table of Contents (TOC) is not exhaustive; the final content may vary. Refer to the sample report for the complete table of contents.
Download Sample PDF to Explore the Key Offerings of the Report @ [https://www.delveinsight.com/sample-request/lewy-body-disease-pipeline-insight?utm_source=abnewswire&utm_medium=market&utm_campaign=kpr]
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email:Send Email [https://www.abnewswire.com/email_contact_us.php?pr=lewy-body-disease-pipeline-assessment-2024-updates-indepth-insights-into-the-clinical-trials-emerging-drugs-latest-approvals-treatment-outlook-companies-annovis-biocervomedvaxxinity]
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Lewy Body Disease Pipeline Assessment, 2024 Updates | In-depth Insights Into the Clinical Trials, Emerging Drugs, Latest Approvals, Treatment Outlook, Companies | Annovis Bio,CervoMed,Vaxxinity here
News-ID: 3508901 • Views: …
More Releases from ABNewswire
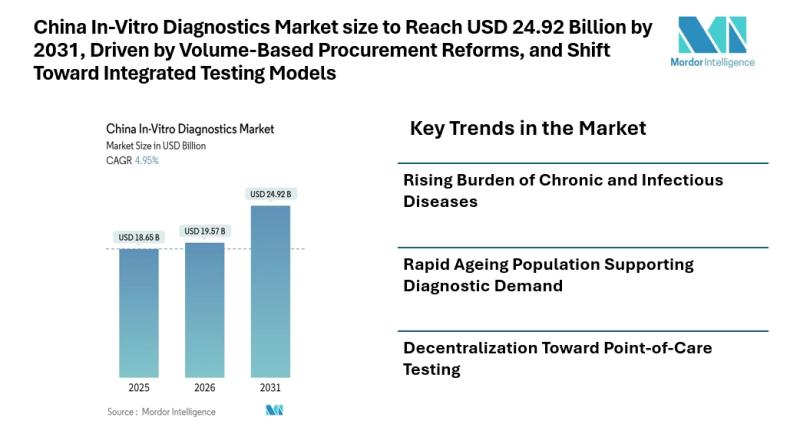
China In-Vitro Diagnostics Market size to Reach USD 24.92 Billion by 2031, Drive …
Mordor Intelligence has published a new report on the china in-vitro diagnostics market, offering a comprehensive analysis of trends, growth drivers, and future projections.
Introduction
According to Mordor Intelligence, the china in-vitro diagnostics market size [https://www.mordorintelligence.com/industry-reports/china-in-vitro-diagnostics-market?utm_source=abnewswire] is projected to reach USD 24.92 billion by 2031, growing from USD 19.57 billion in 2026 at a CAGR of 4.95% during the forecast period. The china in-vitro diagnostics market size reflects steady expansion supported by…

Hyaluronic Acid Market Size to Reach USD 4.07 Billion by 2030 - Mordor Intellige …
Mordor Intelligence has released an in-depth analysis of the hyaluronic acid market, outlining expanding cosmetic, orthopedic, and pharmaceutical applications driving global demand.
Hyaluronic Acid Market Overview
According to Mordor Intelligence, the global hyaluronic acid market size [https://www.mordorintelligence.com/industry-reports/hyaluronic-acid-market?utm_source=abnewswire] reached USD 2.84 billion in 2025 and is projected to grow to USD 4.07 billion by 2030, registering a CAGR of 7.46% during the forecast period.
The strong hyaluronic acid market growth is supported by:
* Increasing…

Scott Bryant Unveils Moon Valley's "Best Value" Listing in Hillcrest East; Signa …
Bryant Real Estate Leverages Data-Driven Performance Metrics to Position New Hillcrest East Property as the Region's Premier Investment Opportunity
PHOENIX, AZ - Scott Bryant, Founder and Team Leader of Bryant Real Estate and a top-performing agent with Keller Williams, has announced the debut of a landmark listing in the Hillcrest East subdivision of Moon Valley. Positioned as "Moon Valley's Best Deal," the property is being introduced at a strategic price point…

Jennifer Rollin Named Best Individual Therapist in Best of Bethesda Awards
Bethesda, MD, USA - Jennifer Rollin, LCSW-C, eating disorder therapist and founder of The Eating Disorder Center, has been named Best Individual Therapist in the 2025 Best of Bethesda Awards. She was selected from among therapists across Montgomery County, Maryland and Upper Northwest Washington, D.C., an honor that reflects both community support and her longstanding commitment to helping individuals recover from eating disorders.
Jennifer Rollin provides eating disorder therapy [https://www.theeatingdisordercenter.com/eatingdisordertherapyrockvilleservices.html] in…
More Releases for Lewy
Lewy Body Dementia (LBD) Market to Reach USD 5.12 Billion by 2034
Pune, India - December 2025 - The global Lewy Body Dementia (LBD) Market, valued at USD 2.98 billion in 2024, is projected to reach USD 5.12 billion by 2034, growing at a 5.6% CAGR (2025-2034), according to Exactitude Consultancy. Rising neurodegenerative disease prevalence, improved diagnostic imaging, and growing awareness of cognitive and behavioral symptoms are driving market expansion.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71909
Market Summary
The Lewy Body…
Lewy Body Dementia Treatment Market Trends That Will Shape the Next Decade: Insi …
Use code ONLINE20 to get 20% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Lewy Body Dementia Treatment Market Size By 2025?
The market for lewy body dementia therapies has experienced robust expansion lately, projected to increase its valuation from $4.43 billion in 2024 to $4.69 billion in 2025, reflecting a compound annual growth rate (CAGR) of 5.8%; this…
Lewy Body Dementia Market to Reach USD 9.4 Billion by 2034
Lewy Body Dementia (LBD) is the second most common form of progressive dementia after Alzheimer's disease, accounting for 10-15% of dementia cases. It is caused by the abnormal accumulation of alpha-synuclein protein (Lewy bodies) in the brain, leading to cognitive decline, visual hallucinations, fluctuating alertness, REM sleep behavior disorder, and Parkinsonism symptoms.
Download Full PDF Sample Copy of Market Report @ https://exactitudeconsultancy.com/request-sample/71909
With aging populations and rising awareness, the LBD market is…
Lewy Body Dementia Market Forecast 2025-2034: Comprehensive Analysis And Growth …
We've updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.
What Is the Projected Growth of the Lewy Body Dementia Market?
In recent times, the market size for Lewy body dementia has witnessed substantial growth. It is projected to increase from $1.12 billion in 2024 to $1.21 billion in 2025, with a compound annual growth rate (CAGR) of 7.5%. The historic growth…
Lewy Body Dementia Treatment Market Challenges: Growth, Share, Value, Trends and …
Lewy Body Dementia Treatment Market Size And Forecast by 2031
The LBD Therapy Market is expanding rapidly, driven by increasing consumer demand, technological advancements, and industry-wide innovation. According to top market research firms, businesses in the Neurodegenerative Disorder Treatment Market are prioritizing digital transformation, product development, and data-driven decision-making to stay competitive. With rising investments in automation and efficiency, the Dementia Care Market is evolving to meet changing customer preferences.…
Lewy Body Dementia Treatment Market Focuses on Advancements in Diagnosis and The …
Global Lewy Body Dementia Treatment Market, By Drug Type (Cholinesterase Inhibitors, Antipsychotic Drugs, Antidepressants, Benzodiazepine, and Modafinil), Application Type (Parkinson's Disease and Alzheimer's Disease), Mode of Purchase (Prescription and Over the Counter), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies and Others) - Industry Trends and Forecast to 2031.
Data Bridge Market Research analyses that the Global Lewy Body Dementia Treatment Market which was USD 5.66 Billion in 2023…
