Press release
Pharmacovigilance Market Size Projected to Reach USD 12.03 Billion By 2031, Driven by Drug Safety Imperatives and Regulatory Stringency
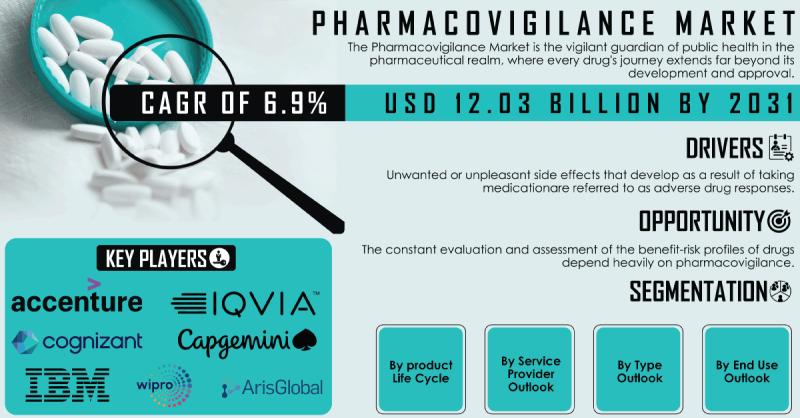
The Pharmacovigilance Market Size was valued at USD 7.05 Billion in 2023 and expected to reach USD 12.03 Billion by 2031 growing at a CAGR of 6.9% over the forecast period of 2024-2031. Propelling this upward market trajectory are the escalating drug safety obligations mandated by regulatory bodies coupled with the pharmaceutical industry's intense focus on proactive safety monitoring to mitigate risks across product lifecycles.Pharmacovigilance encompasses the practice of monitoring, detecting, assessing and preventing adverse effects and other safety-related issues with pharmaceutical products and biological therapies. These critical activities span the entire product lifecycle from preclinical and clinical development through post-marketing surveillance. Regulatory agencies like the FDA and EMA enforce rigorous pharmacovigilance requirements that drug developers and manufacturers must adhere to protect public health.
"As our scientific capabilities to develop increasingly complex therapeutic products expand, our pharmacovigilance systems must keep pace to vigilantly monitor their safety profiles," said Dr. Janet Woodcock, former FDA Commissioner. "Both public health and the reputation of the biopharmaceutical industry hinge on this commitment to vigilant safety monitoring."
Download Free Sample Report of Pharmacovigilance Market @ https://www.snsinsider.com/sample-request/3095
List of Pharmacovigilance Companies Profiled in Report:
• ArisGlobal
• BioClinica Inc.
• Capgemini
• ClinChoice (formerly FMD K&L)
• Clinquest Group B.V.
• Cognizant
• COGNIZANT
• Comp7
• Comp8
• IBM Corporation
• ICON plc.
• IQVIA
• IQVIA
• ITClinical
• Laboratory Corporation of America Holdings
• PAREXEL International Corporation.
• TAKE Solutions Ltd.
• United BioSource LLC
• Wipro Ltd.
(To view Full list of companies, Ask for Sample Report)
Regulatory Mandates and High-Stakes Driving Adoption
The primary driving force behind pharmacovigilance market growth is the expanding scope of regulatory requirements enforced by global health authorities. Over the past two decades, numerous high-profile drug safety crises have catalyzed more stringent pharmacovigilance guidelines encompassing adverse event reporting, signal detection, risk management and enhanced post-marketing surveillance.
For instance, the FDA's Amendments Act (FDAAA) of 2007 established robust post-approval safety monitoring programs like the Sentinel Initiative. Similarly, the EMA has continually evolved its pharmacovigilance legislation with mandates like the 2010 Good Vigilance Practices (GVP) to harmonize safety monitoring across the EU.
Compounding this regulatory pressure is the immense financial burden and brand risk that safety issues pose to drug manufacturers. Serious adverse events and product recalls can decimate a therapy's commercial potential while exposing firms to billions in potential liabilities. According to research firm EvaluatePharma, drug safety crises have cost pharmaceutical companies over $38 billion since the late 1990s.
Consequently, investments in comprehensive, tech-enabled pharmacovigilance systems have become an existential priority for the biopharmaceutical industry to avoid these high-stakes risks. From advanced adverse event databases and signal detection software to AI-powered automated case processing, companies are embracing cutting-edge solutions.
Technological Innovation Driving New Capabilities
While regulatory requirements have expanded pharmacovigilance obligations, innovation across fields like artificial intelligence, natural language processing, cloud computing and data analytics is enhancing the efficiency and efficacy of safety monitoring activities. Machine learning algorithms can rapidly sift through massive volumes of adverse event data to identify potential safety signals that may elude traditional methods.
Similarly, cognitive computing disciplines like NLP are streamlining literature monitoring and label scrutiny by automatically extracting insights from unstructured text sources at scale. Cloud-based pharmacovigilance platforms are also gaining traction by providing secure, globally accessible data aggregation and analytics capabilities.
Major technology giants like IBM, Google, Amazon and Microsoft have invested heavily in computational pharmacovigilance R&D. Their innovations in automation, predictive modeling and intelligent safety intelligence systems are yielding new safety monitoring capabilities while reducing human workloads.
On the services front, an entire industry has emerged to support pharmaceutical companies' pharmacovigilance needs. Specialized providers like BioVigil, Cognizant and others offer end-to-end safety operations management ranging from case processing to risk management programs leveraging software, tools and subject matter expertise.
Have Any Query? Ask Our Experts @ https://www.snsinsider.com/enquiry/3095
Market Segmentation and Outlook
By Product Life Cycle
• Pre-Clinical
• Phase I
• Phase II
• Phase III
• Phase IV
By Service Provider Outlook
• In-house
• Contract Outsourcing
By Type Outlook
• Spontaneous reporting
• Intensified ADR Reporting
• EHE mining
Process Flow Outlook
• Case Data management
• Signal Detection
• Risk Management System
Therapeutic Outlook
• Oncology
• Neurology
• Cardiology
• Respiratory systems
• Others
End Use Outlook
• Pharmaceuticals
• Medical Device manufacturers
• Others
Within the pharmacovigilance market, post-marketing services focused on monitoring and evaluating safety throughout the commercial product lifecycle captured the largest revenue share in 2022. However, the pre-marketing and clinical segments supporting safety activities during drug development are expected to experience the highest growth rates.
From a therapeutic area perspective, the oncology and immunology segments currently dominate due to the elevated safety monitoring requirements associated with novel cancer therapies and biologics. However, neurology, metabolic disorders and rare diseases segments are poised for accelerated growth.
Pharmaceutical companies and biologics manufacturers account for the lion's share of end-user spend on pharmacovigilance solutions and services. However, medical device manufacturers are an emerging customer segment as device monitoring requirements expand globally.
Geographically, North America represents the largest regional market currently driven by stringent U.S. FDA oversight and a concentration of major biopharmaceutical companies. However, Europe and Asia-Pacific are anticipated to experience faster growth rates as regulations harmonize and emerging markets advance their pharmacovigilance capabilities to support domestic drug development initiatives.
Key Takeaways:
• Pharmacovigilance market projected to grow from $7.05 Bn in 2023 to $12.03 Bn by 2031
• 6.9% CAGR driven by expanding regulatory mandates, drug safety monitoring priorities
• Adverse events, product recalls carry immense financial/reputational risks for drugmakers
• Innovation in AI, cloud, NLP enhancing pharmacovigilance capabilities
• Outsourced services and software solutions segment rapidly growing
• Oncology and immunology current largest therapeutic areas; high growth in neurology
• North America largest region but Europe, APAC to grow faster aligned with regulations
Purchase Pharmacovigilance Market Report @ https://www.snsinsider.com/checkout/3095
As biopharmaceutical pipelines swell with increasingly complex and high-risk therapies, robust pharmacovigilance will remain an indispensable obligation and strategic priority for life sciences companies globally. While financial and operational challenges exist, the combination of stringent regulatory enforcement and existential brand threats will ultimately compel comprehensive adoption and continued investment in this domain. Pharmacovigilance is positioned for consistent growth as a mission-critical function safeguarding public health.
Table of Content
Chapter 1 Introduction
Chapter 2 Research Methodology
Chapter 3 Pharmacovigilance Market Dynamics
Chapter 4 Impact Analysis (COVID-19, Ukraine- Russia war, Ongoing Recession on Major Economies)
Chapter 5 Value Chain Analysis
Chapter 6 Porter's 5 forces model
Chapter 7 PEST Analysis
Chapter 8 Pharmacovigilance Market Segmentation, By Product Life Cycle
Chapter 9 Pharmacovigilance Market Segmentation, By Service Provider Outlook
Chapter 10 Pharmacovigilance Market Segmentation, By Type Outlook
Chapter 11 Pharmacovigilance Market Segmentation, By Process Flow Outlook
Chapter 12 Pharmacovigilance Market Segmentation, By Therapeutic Outlook
Chapter 13 Pharmacovigilance Market Segmentation, By End Use Outlook
Chapter 14 Regional Analysis
Chapter 15 Company profile
Chapter 16 Competitive Landscape
Chapter 17 Use Case and Best Practices
Chapter 18 Conclusion
Continued…
Browse Pharmacovigilance Market Report @ https://www.snsinsider.com/reports/pharmacovigilance-market-3095
Contact Us:
Akash Anand - Head of Business Development & Strategy
info@snsinsider.com
Phone: +1-415-230-0044 (US) | +91-7798602273 (IND)
About US:
SNS Insider has been a leader in data and analytics globally with its authentic consumer and market insights. The trust of our clients and business partners has always been at the center of who we are as a company. We are a business that leads the industry in innovation, and to support the success of our clients, our highly skilled engineers, consultants, and data scientists have consistently pushed the limits of the industry with innovative methodology and measuring technologies.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance Market Size Projected to Reach USD 12.03 Billion By 2031, Driven by Drug Safety Imperatives and Regulatory Stringency here
News-ID: 3501830 • Views: …
More Releases from SNS Insider

Private Cloud Services Market Forecast Predicts Promising Growth Ahead
Private Cloud Services Market Scope and Overview
The Private Cloud Services Market has witnessed significant growth in recent years, fueled by the increasing adoption of cloud computing technologies across various industries. Private clouds offer enhanced security, control, and customization compared to public cloud services, making them a preferred choice for enterprises seeking to leverage cloud capabilities while maintaining data sovereignty and compliance. This report delves into the competitive landscape, market segmentation,…
Video Surveillance Market Analysis Unveils Insights for Growth and Development
Video Surveillance Market Scope and Overview
The Video Surveillance Market has seen significant growth over the past few decades, driven by advancements in technology and an increasing need for security across various sectors. Video surveillance systems, once primarily used for security purposes, have now expanded their applications to include monitoring, analysis, and even preventive measures in various industries. This report provides a comprehensive analysis of the video surveillance market, covering its…
Electric Scooter Battery Market Charges Ahead, Propelling Sustainable Urban Mobi …
The Global Electric Scooter Battery Market is experiencing a remarkable surge, fueled by the rising demand for eco-friendly and convenient transportation solutions in urban environments. As cities around the world grapple with traffic congestion, air pollution, and the need for sustainable mobility, the electric scooter battery market is poised to play a pivotal role in shaping the future of urban transportation. According to a comprehensive market research report, the electric…

Solar-Powered Vehicle Market Accelerates Towards a Sustainable Future, Projected …
The Global Solar-Powered Vehicle Market is rapidly gaining momentum, driven by the urgent need to combat climate change and reduce greenhouse gas emissions. As the world transitions towards a greener and more sustainable future, the adoption of solar-powered vehicles is emerging as a game-changer in the automotive industry. According to a comprehensive market research report, the solar-powered vehicle market, valued at $1.27 billion in 2023, is expected to reach a…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…