Press release
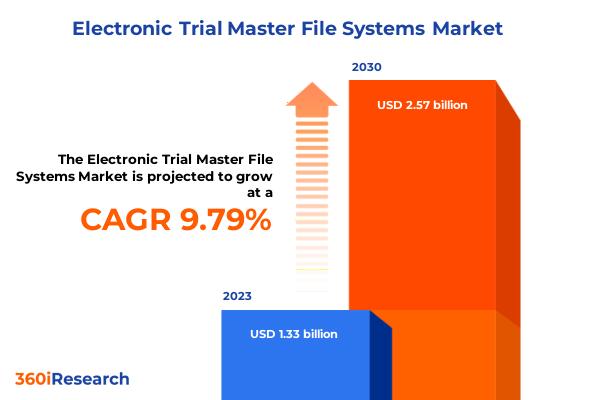
Electronic Trial Master File Systems Market worth $2.57 billion by 2030, growing at a CAGR of 9.79% - Exclusive Report by 360iResearch
The "Electronic Trial Master File Systems Market by Component (Services, Software), Distribution (On-Cloud, On-Premise), End-User - Global Forecast 2024-2030" report has been added to 360iResearch.com's offering.The Global Electronic Trial Master File Systems Market to grow from USD 1.33 billion in 2023 to USD 2.57 billion by 2030, at a CAGR of 9.79%.
Request a Free Sample Report @ https://www.360iresearch.com/library/intelligence/electronic-trial-master-file-systems?utm_source=openpr&utm_medium=referral&utm_campaign=sample
The electronic trial master file (eTMF) leverages software and server technology to establish, collect, track, and archival essential clinical study documents. The eTMF systems are convenient for managing the large data pool collected during the trials with minimal error and no human involvement. The eTMF offers a standardized procedure for classifying and keeping track of files, photographs, and other digital content for clinical trials required for compliance with government regulatory agencies. The increasing number of clinical trials and several government initiatives for the digitalization of the healthcare sector is significantly increasing the adoption of eTMF systems with the growing need for data management and storage of documents. However, the high cost associated with installing eTMF systems and concerns regarding medical data privacy impedes the adoption of eTMF systems. In addition, to cope with increasing clinical trials, companies focus more on bringing advanced and easy-to-use software to reduce the load of huge data generated through clinical trials.
The adoption of healthcare information technology is increasing in the United States, augmenting the need for electronic trial master file software to ease the work of huge data generated through clinical trials every year. The huge number of clinical trials taking place and increasing R&D expenditure by pharmaceutical companies are beneficial as there is a need for a suitable tool to help researchers keep track of data and store the data effectively. According to the Pharmaceutical Researchers and Manufacturers of America (PhRMA), in 2022, R&D expenditures belonging to biopharmaceutical firms were approximately USD 80 billion in the United States. In North America, increased outsourcing of clinical trials and the need for better compliance and data security measures increase the adoption of electronic trial master file (eTMF) systems. In Eastern Europe, the eTMF market is expected to grow in the coming years owing to the adoption of advanced analytics and AI technologies, increased focus on patient-centricity, and emerging markets. In Asia-Pacific, increasing outsourcing of clinical trials and the adoption of advanced technologies raised the utilization of eTMF systems.
Market Segmentation & Coverage:
This research report categorizes the Electronic Trial Master File Systems Market in order to forecast the revenues and analyze trends in each of following sub-markets:
Based on Component, market is studied across Services and Software. The Services is projected to witness significant market share during forecast period.
Based on Distribution, market is studied across On-Cloud and On-Premise. The On-Cloud is projected to witness significant market share during forecast period.
Based on End-User, market is studied across Contract Research Organizations and Pharmaceutical & Biotechnology Companies. The Contract Research Organizations is projected to witness significant market share during forecast period.
Based on Region, market is studied across Americas, Asia-Pacific, and Europe, Middle East & Africa. The Americas is further studied across Argentina, Brazil, Canada, Mexico, and United States. The United States is further studied across California, Florida, Illinois, New York, Ohio, Pennsylvania, and Texas. The Asia-Pacific is further studied across Australia, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Taiwan, Thailand, and Vietnam. The Europe, Middle East & Africa is further studied across Denmark, Egypt, Finland, France, Germany, Israel, Italy, Netherlands, Nigeria, Norway, Poland, Qatar, Russia, Saudi Arabia, South Africa, Spain, Sweden, Switzerland, Turkey, United Arab Emirates, and United Kingdom. The Asia-Pacific is projected to witness significant market share during forecast period.
Inquire Before Buying @ https://www.360iresearch.com/library/intelligence/electronic-trial-master-file-systems?utm_source=openpr&utm_medium=referral&utm_campaign=inquire
FPNV Positioning Matrix:
The FPNV Positioning Matrix is essential for assessing the Electronic Trial Master File Systems Market. It provides a comprehensive evaluation of vendors by examining key metrics within Business Strategy and Product Satisfaction, allowing users to make informed decisions based on their specific needs. This advanced analysis then organizes these vendors into four distinct quadrants, which represent varying levels of success: Forefront (F), Pathfinder (P), Niche (N), or Vital(V).
Market Share Analysis:
The Market Share Analysis offers an insightful look at the current state of vendors in the Electronic Trial Master File Systems Market. By comparing vendor contributions to overall revenue, customer base, and other key metrics, we can give companies a greater understanding of their performance and what they are up against when competing for market share. The analysis also sheds light on just how competitive any given sector is about accumulation, fragmentation dominance, and amalgamation traits over the base year period studied.
Key Company Profiles:
The report delves into recent significant developments in the Electronic Trial Master File Systems Market, highlighting leading vendors and their innovative profiles. These include Advarra, Inc., Anju Software, Inc., ArborSys Group, ArisGlobal LLC, Aurea, Inc., Cereblis LLC, Clario by eResearchTechnology GmbH, Clinblocks B.V., Clinevo Technologies Private Limited, Cloudbyz, Inc., ComplianceQuest, Crucial Data Solutions, Inc., Daffodil Software Private Limited, Dassault Systèmes, DataRiver S.r.l., Dell Technologies, Inc., Ennov SAS, ethica CRO Inc., EvidentIQ Group GmbH, Flex Databases s.r.o., Florence Healthcare, Inc., Freyr Solutions, ICON PLC, IKCON PHARMA Inc., IQVIA Inc., Kivo, Inc., Laboratory Corporation of America Holdings, MasterControl Solutions, Inc., McDougall Scientific Ltd., Medrio, Inc., Montrium Inc., Navitas Life Sciences, Novotech Health Holdings, Octalsoft, OpenClinica, LLC, Oracle Corporation, PHARMASEAL, Phlexglobal Ltd. by PharmaLex Group, Prevail Infoworks, Inc., Questex LLC, Sarjen Systems Pvt. Ltd., SGS Société Générale de Surveillance SA, SimpleTrials, SMART-TRIAL ApS, SureClinical Inc., TCell Clinical Services, Techsol Corporation, TransPerfect Translations GmbH, Veeva Systems Inc., and Vial Health Technology, Inc..
Key Topics Covered:
1. Preface
2. Research Methodology
3. Executive Summary
4. Market Overview
5. Market Insights
6. Electronic Trial Master File Systems Market, by Component
7. Electronic Trial Master File Systems Market, by Distribution
8. Electronic Trial Master File Systems Market, by End-User
9. Americas Electronic Trial Master File Systems Market
10. Asia-Pacific Electronic Trial Master File Systems Market
11. Europe, Middle East & Africa Electronic Trial Master File Systems Market
12. Competitive Landscape
13. Competitive Portfolio
The report provides insights on the following pointers:
1. Market Penetration: Provides comprehensive information on the market offered by the key players
2. Market Development: Provides in-depth information about lucrative emerging markets and analyzes penetration across mature segments of the markets
3. Market Diversification: Provides detailed information about new product launches, untapped geographies, recent developments, and investments
4. Competitive Assessment & Intelligence: Provides an exhaustive assessment of market shares, strategies, products, certification, regulatory approvals, patent landscape, and manufacturing capabilities of the leading players
5. Product Development & Innovation: Provides intelligent insights on future technologies, R&D activities, and breakthrough product developments
The report answers questions such as:
1. What is the market size and forecast of the Electronic Trial Master File Systems Market?
2. Which are the products/segments/applications/areas to invest in over the forecast period in the Electronic Trial Master File Systems Market?
3. What is the competitive strategic window for opportunities in the Electronic Trial Master File Systems Market?
4. What are the technology trends and regulatory frameworks in the Electronic Trial Master File Systems Market?
5. What is the market share of the leading vendors in the Electronic Trial Master File Systems Market?
6. What modes and strategic moves are considered suitable for entering the Electronic Trial Master File Systems Market?
Read More @ https://www.360iresearch.com/library/intelligence/electronic-trial-master-file-systems?utm_source=openpr&utm_medium=referral&utm_campaign=analyst
Contact 360iResearch
Mr. Ketan Rohom
Sales & Marketing,
Office No. 519, Nyati Empress,
Opposite Phoenix Market City,
Vimannagar, Pune, Maharashtra,
India - 411014.
sales@360iresearch.com
+1-530-264-8485
+91-922-607-7550
About 360iResearch
360iResearch is a market research and business consulting company headquartered in India, with clients and focus markets spanning the globe.
We are a dynamic, nimble company that believes in carving ambitious, purposeful goals and achieving them with the backing of our greatest asset - our people.
Quick on our feet, we have our ear to the ground when it comes to market intelligence and volatility. Our market intelligence is diligent, real-time and tailored to your needs, and arms you with all the insight that empowers strategic decision-making.
Our clientele encompasses about 80% of the Fortune Global 500, and leading consulting and research companies and academic institutions that rely on our expertise in compiling data in niche markets. Our meta-insights are intelligent, impactful and infinite, and translate into actionable data that support your quest for enhanced profitability, tapping into niche markets, and exploring new revenue opportunities.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Electronic Trial Master File Systems Market worth $2.57 billion by 2030, growing at a CAGR of 9.79% - Exclusive Report by 360iResearch here
News-ID: 3437060 • Views: …
More Releases from 360iResearch

Rising Incidence of Human Metapneumovirus in Vulnerable Populations Boosts Globa …
In recent years, the silent ascent of human metapneumovirus (HMPV) infections has begun to capture significant attention within the realm of infectious diseases. As we advance in medical science, unraveling complexities of age-old pathogens like common influenza or emerging illnesses like COVID-19, a critical discourse has been emerging around HMPV. Particularly, there seems to be a burgeoning acknowledgment of its growing impact on vulnerable global populations, propelling an increased demand…

The Meat Alternatives Market size was estimated at USD 9.39 billion in 2023 and …
From Appetite to Advocacy: The Rising Demand for Meat Alternatives
In recent years, the global food industry has been undergoing a remarkable transformation, driven primarily by an increasing consumer demand for healthier, sustainable, and ethically sourced food products. This seismic shift has brought traditional meat alternatives and high-protein plant-based foods into the spotlight. As the world becomes more conscious of the implications of meat consumption on health and the environment, the…

The Mobility-as-a-Service Market size was estimated at USD 264.80 billion in 202 …
Unpacking the Surge in Investments and Collaborations to Bolster Mobility-as-a-Service
In recent years, as urban landscapes continually evolve, a transformative shift known as Mobility-as-a-Service (MaaS) has reshaped the way we perceive transportation. Marked by the integration of various forms of transport services into a single accessible on-demand mobility solution, MaaS is rapidly gaining traction across global cities. As an emerging paradigm, it's not just shaping the future of travel but also…

The Data Center Services Market size was estimated at USD 56.65 billion in 2023 …
Smart City Revolutions: Why Data Center Colocation is the Future Backbone
In the era of digital transformation, urban landscapes across the globe are undergoing a seismic shift toward becoming "smart cities." The concept of a smart city revolves around using digital technology, IoT (Internet of Things), AI, and data analytics at an unprecedented scale to improve urban infrastructure, manage resources efficiently, and enhance the quality of life for citizens. A vital…
More Releases for Trial
Clinical Trial Investigative Site Network Market Clinical Trial Investigative Si …
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trial Investigative Site Network Market - (By Therapeutic Areas (Oncology, Cardiology, CNS, Pain Management, Endocrine, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By End-use (Sponsor, CRO)), Trends, Industry Competition Analysis, Revenue and Forecast To 2034."
According to the latest research by InsightAce Analytic, the Global Clinical Trial Investigative Site Network Market…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Transformative Trends Impacting the Electronic Trial Master File (eTMF) Systems …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Electronic Trial Master File (eTMF) Systems Market Size By 2025?
The market size of the electronic trial master file (eTMF) systems has experienced fast growth over recent years. The market is projected to increase from $1.36 billion in 2024 to $1.55 billion in 2025, with…
Clinical Trial Imaging market
The Clinical Trial Imaging market crossed the US$ 1.09 billion mark in 2022 and is expected to hit US$ 1.94 billion by 2030, recording a CAGR of 7.5% during the forecast period.
Rising R&D spending, a rapidly growing pharmaceutical industry, and an increase in the number of contract research organizations are some of the major factors driving the market's growth. There has been an increase in pharmaceutical companies due to the…
Clinical Trial Logistics
Clinical Trial Logistics
16th to 17th May 2011, Marriott Regents Park, London, United Kingdom.
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical trials…
Clinical Trial Logistics
Announcing SMi's 5th annual…
Clinical Trial Logistics conference
16th and 17th May 2011, Central London, UK
www.smi-online.co.uk/2011logistics-london6.asp
It currently costs just over £500 million ($800 million) to bring a new chemical to market and development timelines continue to fall in the 10-15 year range. A key reason for high R&D costs is due to logistical failures including failure to recruit patients on time. A way to avoid this is to move clinical…