Press release
IVD Contract Manufacturing Services Market to be Worth $25.80 Billion by 2031
According to a new market research report, 'IVD Contract Manufacturing Services Market by Type (Assay Development, Manufacturing), Category (Reagents, Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, Urinalysis)-Global Forecast to 2031', published by Meticulous Research®, the IVD contract manufacturing services market is projected to reach $25.80 billion by 2031, at a CAGR of 7.8% from 2024 to 2031.IVD contract manufacturing services allow organizations to outsource manufacturing processes. Companies outsource a product or a part of a product. This is often used to increase production rates or to expand product offerings without investing in new equipment and machinery.
The growth of the IVD contract manufacturing services market is driven by several factors, including the rising prevalence of infectious diseases, a shift in focus from centralized laboratories to point-of-care testing services, regulatory complexities faced by IVD companies, and the need for cost-effective manufacturing of IVD tests. Factors such as high economic growth and increased outsourcing in emerging countries are opportunities that would help grow the market in the future. The lack of skilled professionals could be considered a challenge for the IVD contract manufacturing services market. However, maintaining product quality and protection of proprietary information restrains the growth of the market.
The report also includes an extensive assessment of the product portfolio, geographic analysis, and key growth strategies adopted by leading market players in the last three to four years. In recent years, the IVD contract manufacturing services market has witnessed several organic and inorganic strategic developments. The key players profiled in the IVD contract manufacturing services market report are Cenogenics Corporation (U.S.), In-Vitro Diagnostic Developers, Inc. (IDxDI) (U.S.), Savyon Diagnostics (Israel), KMS Systems, Inc. (U.S.), Nova Biomedical (U.S.), LRE Medical (Germany), Cone Bioproducts (U.S.), Invetech, Inc. (Australia), Avioq, Inc. (U.S.), TCS Biosciences Ltd. (U.K.), Affinity Life Sciences, Inc. (U.S.), Coris BioConcept (Belgium), Meridian Bioscience, Inc. (U.S.), Affinity Biologicals, Inc. (Canada), Biokit S.A. (Spain), Merck KgaA (Germany), Thermo Fisher Scientific, Inc. (U.S.), and Maxim Biomedical, Inc. (U.S.).
Download Free PDF Sample Copy of the Report: https://www.meticulousresearch.com/download-sample-report/cp_id=5226
Regulatory Complexities Faced by IVD Companies
Although IVD holds excellent potential for early diagnosis, the regulatory approval processes for these devices or tests are tedious. Regulatory approvals include a review of the clinical data, calibration, manufacturing methods, quality control, risk assessment, and other factors to ensure the reproducibility and safety of the devices/tests. Despite this, the primary regulatory hurdle for diagnostic devices is complexity. The monitoring and controlling of the quality of components and assembly during the transition of diagnostic assays to full-scale production is important. Also, supply chain management and risk mitigation strategies become critical. To keep the final assay product compliant and sellable, quality management systems (QMS) and careful documentation of production processes according to ISO and other regulatory guidelines become necessary. To verify safety and quality compliance, government bodies conduct scheduled and unscheduled audits of all manufacturing facilities.
Manufacturers found that developing tests for point-of-care settings is difficult compared to laboratory tests due to the high standards of the FDA. For instance, the FDA determines CLIA status through a point scoring system (1-3, low to high complexity). Devices with a cumulative score greater than 12 are categorized as high-complexity devices. Devices with an aggregate score of less than 12 are classified as moderate-complexity devices. Low-complexity tests can be used in any POC setting due to the low risk of enormous results. On the other hand, it is mandated by the FDA that high and moderate complexity tests must be performed in CLIA-certified labs by qualified personnel. Thus, manufacturers find the bar high and, hence, difficult to develop POC tests.
IVD companies are trying to optimize resources due to regulatory complexities, prompting industry participants to adopt innovative, integrated business models. In such situations, contract manufacturing services are positioning themselves as one-stop-shop solution providers. Their services help IVD companies overcome regulatory complexities, thus driving market growth.
The IVD contract manufacturing services market is segmented by Type (Assay Development Services, Manufacturing Services, and Other Services), Category (Reagents & Consumables and Instruments & Systems), Technology (Immunoassay, Molecular Diagnostics, Clinical Chemistry, Hematology, Microbiology, and Urinalysis), and Geography. The study also evaluates industry competitors and analyzes the regional and country-level markets.
Based on type, in 2024, the manufacturing services segment is expected to account for the largest share of the market. Manufacturing services are FDA-registered and include ISO-certified manufacturing facilities that allow them to provide everything, from small-scale pilot batch manufacture to full commercial production. These facilities carry out full project and production management, quality and regulatory support, and flexibility in scale and process, which results in an increased demand for manufacturing services, hence driving the growth of the segment.
Based on category, in 2024, the reagents & consumables segment is expected to account for the largest share of the market. Reagents & consumables provide high standards of innovation and quality in R&D and manufacturing of IVD assays. This results in laboratory efficiency and better clinical outcomes for patients, therefore driving the growth of the market. Apart from this, the rising prevalence of infectious diseases and the growing demand for POC diagnostic kits also consequently increase the use of reagents & consumables used in diagnosis.
You can Buy Report from Here: https://www.meticulousresearch.com/Checkout/62703221
Based on technology, in 2024, the immunoassay segment is expected to account for the largest share of the market. Immunoassay technologies utilize antigen-antibody reactions for the detection of causative agents. Radioimmunoassay and ELISA are the most widely used immunoassay techniques for the diagnosis of infectious diseases. These tests are used frequently for immunodiagnostics owing to their inherent specificity, high throughput, and the emergence of advanced diagnostic immunoassay formats. Furthermore, the increasing use of immunoassays in Point of Care (PoC) infectious disease testing and the rising trend of automation in immunoassay instruments is propelling the growth of this segment.
Based on geography, in 2024, the market in North America is expected to account for the largest share of the market. The growth of this regional market is primarily driven by the rising prevalence of various infectious diseases, the growing healthcare sector, increasing awareness regarding early disease diagnosis, the growing adoption of advanced diagnostic products, and increasing funding activities coupled with novel advanced diagnostic technologies.
Request For Customization: https://www.meticulousresearch.com/request-customization/cp_id=5226
Scope of the Report:
IVD Contract Manufacturing Services Market-by Type
• Manufacturing Services
• Assay Development Services
• Other Services
(Other Services primarily include product design and lyophilization)
IVD Contract Manufacturing Services Market-by Category
• Reagents & Consumables
• Instruments & Systems
IVD Contract Manufacturing Services Market-by Technology
• Immunoassay
• Molecular Diagnostics
• Clinical Chemistry
• Hematology
• Microbiology
• Urinalysis
IVD Contract Manufacturing Services Market-by Geography
• North America
o U.S.
o Canada
• Europe
o Germany
o France
o U.K.
o Italy
o Spain
o Rest of Europe (RoE)
• Asia-Pacific
o China
o Japan
o India
o Rest of APAC (RoAPAC)
• Latin America
• Middle East & Africa
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/ivd-contract-manufacturing-services-market-5226
About Us
We are the trusted research partners for leading businesses around the world, providing market intelligence focused towards building revenue transformation strategies. Our research is used by Fortune 500 organizations to attain success by scouting next generation revenue opportunities well ahead of their competition.
Address
Office No-202, 203,204,205,206
2nd Floor, Pushpak Business Hub,
Wakad, Pimpri-Chinchwad,
411057, India.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release IVD Contract Manufacturing Services Market to be Worth $25.80 Billion by 2031 here
News-ID: 3435787 • Views: …
More Releases from Meticulous Research®
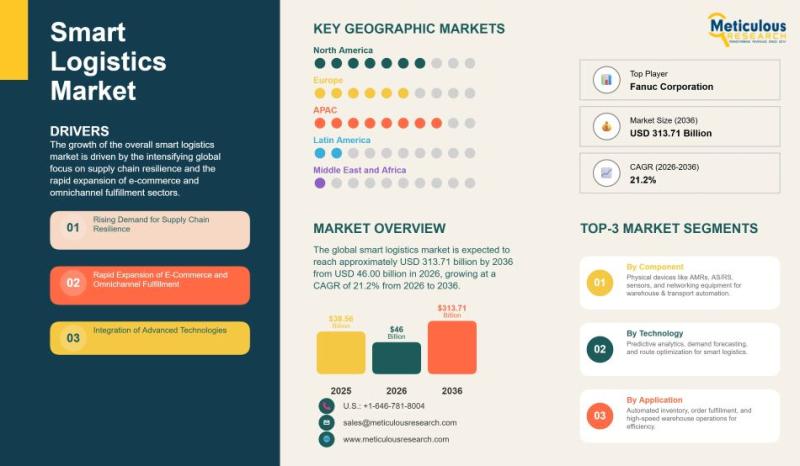
Global Smart Logistics Market Size, Share, Growth Analysis, and Forecast (2026-2 …
The global smart logistics market has emerged as one of the fastest-growing segments within the broader logistics and supply chain ecosystem. In 2025, the market was valued at USD 38.56 billion, reflecting strong adoption of digital technologies across warehousing, transportation, and inventory management operations. The market is projected to expand significantly, reaching approximately USD 46.00 billion in 2026 and further accelerating to USD 313.71 billion by 2036. This impressive expansion…
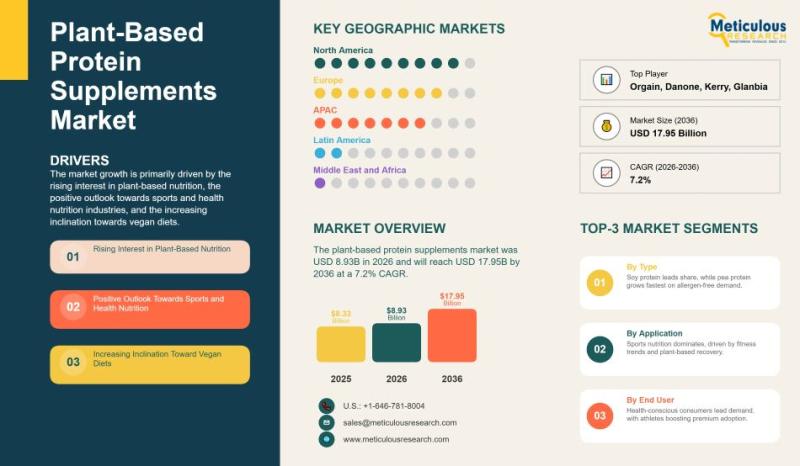
Global Plant-Based Protein Supplements Market: Size, Trends, Growth Analysis, an …
The global plant-based protein supplements market is experiencing consistent and long-term growth, reflecting a significant shift in consumer dietary preferences and health awareness. In 2026, the market was valued at USD 8.93 billion and is projected to reach USD 17.95 billion by 2036, expanding at a compound annual growth rate of 7.2% during the forecast period. This steady growth underscores the increasing integration of plant-based protein products into mainstream nutrition.…
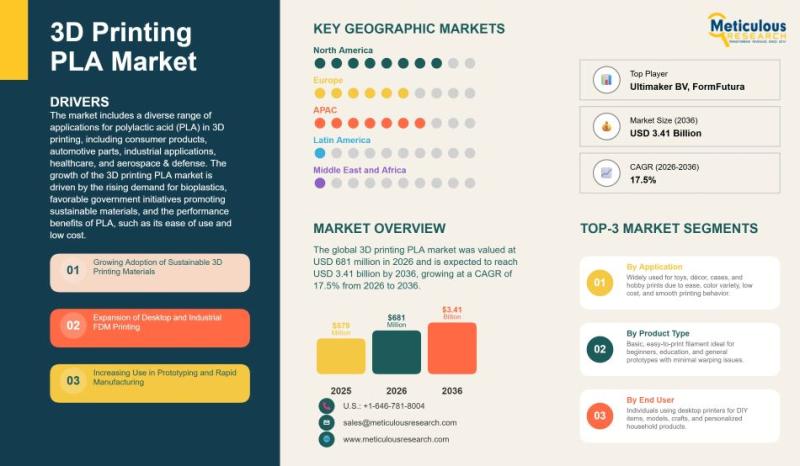
Global 3D Printing PLA Market Size, Growth Trends, and Forecast (2026-2036)
The global 3D printing PLA market has established itself as one of the most dynamic segments within the additive manufacturing materials industry. Valued at USD 681 million in 2026, the market is projected to reach USD 3.41 billion by 2036, registering a strong compound annual growth rate (CAGR) of 17.5% during the forecast period from 2026 to 2036. This rapid expansion reflects the increasing adoption of polylactic acid (PLA) across…
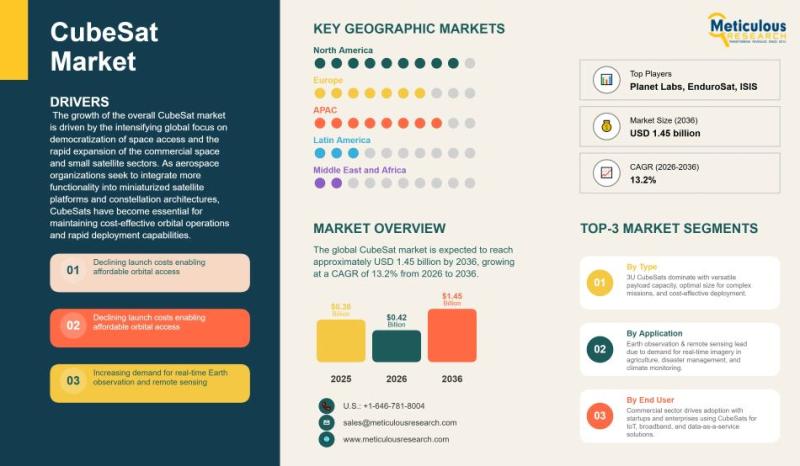
Global CubeSat Market Size, Growth Trends, and Forecast (2026-2036)
The global CubeSat market stood at USD 0.38 billion in 2025 and is projected to expand steadily over the next decade. In 2026, the market size is expected to reach USD 0.42 billion, and by 2036 it is forecast to approach USD 1.45 billion. This reflects a compound annual growth rate of 13.2% during the 2026-2036 period. Growth is largely tied to the broader shift in the space industry toward…
More Releases for IVD
Transformative Trends Impacting the Cancer In Vitro Diagnostics (IVD) Market Lan …
Use code ONLINE30 to get 30% off on global market reports and stay ahead of tariff changes, macro trends, and global economic shifts.
How Large Will the Cancer In Vitro Diagnostics (IVD) Market Size By 2025?
The market size for cancer in vitro diagnostics (IVD) has seen significant growth in the past few years. The market value, which is expected to be $13.36 billion in 2024, is projected to increase to $14.32…
In Vitro Diagnostics (IVD) Market
With the watchful use of established and advanced tools such as SWOT analysis and Porter's Five Forces Analysis, this market report has been structured. While preparing this In Vitro Diagnostics (IVD) Market research report, few of the attributes that have been adopted include highest level of spirit, practical solutions, committed research and analysis, innovation, integrated approaches, and most up-to-date technology.
Every possible effort has been taken while researching and analysing…
Companion Animal IVD Market - Guiding the Path to Optimal Health: Empowering Vet …
Newark, New Castle, USA - new report, titled Companion Animal IVD Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Companion Animal IVD market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Companion Animal IVD market. The report offers an overview of…
IVD Market 2021 | Detailed Report
The IVD market research report delivers accurate data and innovative corporate analysis, helping organizations of all sizes make appropriate decisions. The IVD report also incorporates the current and future global market outlook in the emerging and developed markets. Moreover, the report also investigates regions/countries expected to witness the fastest growth rates during the forecast period.
The IVD research report also provides insights of different regions that are contributing market growth.…
Liquid Biopsy IVD Market 2021 | Detailed Report
According to Market Study Report, Liquid Biopsy IVD Market provides a comprehensive analysis of the Liquid Biopsy IVD Market segments, including their dynamics, size, growth, regulatory requirements, competitive landscape, and emerging opportunities of global industry. An exclusive data offered in this report is collected by research and industry experts team.
Get Free Sample PDF (including full TOC, Tables and Figures) of Liquid Biopsy IVD Market @ https://www.reportsnreports.com/contacts/requestsample.aspx?name=4623688
The report provides a…
Asia IVD Market
According to a new report published by Allied Market Research, the Asia Pacific In-vitro diagnostics market was valued at $12.9 billion in 2015, and is expected to reach $19.0 billion registering a CAGR of 5.6% during 2016 to 2022. The report offers a detailed analysis of the key segments, top investment pockets, changing dynamics, market size & estimations, and competitive scenario.
Download Free Sample Report @ https://www.alliedmarketresearch.com/request-sample/1256
The Asia-Pacific IVD market is…
