Press release
Regulatory Affairs Outsourcing Market Size is Anticipated to Boost at a CAGR of 19 % by 2028 | Transparency Market Research
Regulatory Affairs Outsourcing offers companies several benefits, including access to specialized expertise, cost savings, flexibility, and scalability. By outsourcing regulatory functions to external service providers, companies can leverage the knowledge and experience of regulatory professionals without the need to maintain an in-house regulatory affairs team. This allows companies to focus on their core competencies, accelerate product development timelines, and navigate regulatory challenges more efficiently.Regulatory Affairs Outsourcing market is estimated to attain a valuation of US$ 17.3 Bn by the end of 2028, states a study by Transparency Market Research (TMR). Besides, the report notes that the market is prognosticated to expand at a CAGR of 19% during the forecast period, 2021-2028
Get a Sample Copy of the Regulatory Affairs Outsourcing Market Research Report -https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=3528&utm_source=openpr_amitugare&utm_medium=openpr
The significant players operating in the global Regulatory Affairs Outsourcing market are
Accell Clinical Research, LLC, Charles River Laboratories International, Inc., Clinilabs, Inc., Covance, Inc., Criterium, Inc., ICON plc, Medpace, Inc., PAREXEL International Corporation, Pharmaceutical Product Development, (PPD) LLC, Promedica International, Quintiles Transnational Corporation, and WuXi App Tec.
Key Drivers of Regulatory Affairs Outsourcing:
Complex Regulatory Landscape: The global regulatory environment for healthcare products is complex and constantly evolving, with multiple regulatory agencies, guidelines, and requirements that vary by region and product type. Outsourcing regulatory affairs allows companies to access specialized knowledge and keep pace with regulatory changes without the need for extensive internal resources.
Cost Containment: Outsourcing regulatory affairs can be cost-effective compared to maintaining an in-house regulatory team, particularly for small and medium-sized companies with limited resources. External service providers often offer flexible pricing models, allowing companies to scale regulatory activities according to their needs and budgets.
Access to Expertise: Regulatory affairs outsourcing provides access to regulatory experts with specialized knowledge and experience in specific therapeutic areas, product types, or regions. These experts can provide strategic guidance, regulatory intelligence, and practical insights to navigate complex regulatory pathways and optimize regulatory outcomes.
Time Efficiency: Outsourcing regulatory functions can accelerate product development timelines by streamlining regulatory processes, reducing administrative burdens, and facilitating timely submissions and approvals. External service providers can dedicate resources to regulatory activities, allowing internal teams to focus on other priorities.
Risk Mitigation: Regulatory affairs outsourcing can help companies mitigate regulatory risks by ensuring compliance with applicable laws, regulations, and quality standards. External service providers can conduct regulatory assessments, gap analyses, and compliance audits to identify potential issues and implement corrective actions proactively.
Recent Developments in Regulatory Affairs Outsourcing:
Increased adoption of outsourcing models by pharmaceutical, biotechnology, medical device, and diagnostics companies to streamline regulatory operations, optimize resource allocation, and enhance regulatory compliance.
Expansion of regulatory affairs outsourcing services to include specialized areas such as pharmacovigilance, medical writing, regulatory intelligence, market access, and regulatory technology (RegTech) solutions.
Growth of the regulatory affairs outsourcing market globally, driven by the globalization of clinical trials, the harmonization of regulatory requirements, and the increasing complexity of product development pathways.
Integration of digital technologies, data analytics, and automation tools into regulatory affairs outsourcing services to improve efficiency, transparency, and collaboration between companies and external service providers.
Collaboration between regulatory affairs outsourcing providers, industry associations, regulatory agencies, and academia to address emerging regulatory challenges, promote best practices, and advance regulatory science and innovation.
Buy this Premium Research Report: - https://www.transparencymarketresearch.com/checkout.php?rep_id=3528<ype=S
Market Segmentation -
Service
Regulatory Affairs
Clinical Trial Applications and Product Registrations
Regulatory Writing and Publishing
Regulatory Consulting and Legal Representation
Others
This Report lets you identify the opportunities in Regulatory Affairs Outsourcing Market by means of a region:
North America (the United States, Canada, and Mexico)
Europe (Germany, UK, France, Italy, Russia, Turkey, etc.)
Asia-Pacific (China, Japan, Korea, India, Australia, and Southeast Asia (Indonesia, Thailand, Philippines, Malaysia, and Vietnam))
South America (Brazil etc.) The Middle East and Africa (North Africa and GCC Countries)
Key Features of the Regulatory Affairs Outsourcing Market Report: -
➤ Analyze competitive developments such as expansions, deployments, new product launches, and market acquisitions.
➤ Examine the market opportunities for stakeholders by identifying higher growth sections.
➤ To study and analyze the global Regulatory Affairs Outsourcing industry status and forecast including key regions.
➤ An in-depth analysis of key product segments and application spectrum, providing strategic recommendations to incumbents and new entrants to give them a competitive advantage over others.
➤ It provides a comprehensive analysis of key regions of the industry as well as a SWOT analysis and Porter's Five Forces analysis to provide a deeper understanding of the market.
➤ It helps you make strategic business decisions and investment plans.
More Trending Reports by Transparency Market Research -
Medical Membrane Market: https://www.globenewswire.com/en/news-release/2024/03/07/2842005/32656/en/Medical-Membrane-Market-Size-Share-to-Surpass-USD-10-2-billion-by-2031-Analysis-by-Transparency-Market-Research-Inc.html
Stem Cell Manufacturing Market: https://www.globenewswire.com/en/news-release/2024/03/07/2842010/32656/en/Stem-Cell-Manufacturing-Market-Worth-USD-26-6-billion-Growing-At-9-2-CAGR-by-2033-Report-By-Transparency-Market-Research-Inc.html
About Us Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
𝐂𝐨𝐧𝐭𝐚𝐜𝐭 𝐔𝐬
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA - Canada Toll Free: 866-552-3453
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Regulatory Affairs Outsourcing Market Size is Anticipated to Boost at a CAGR of 19 % by 2028 | Transparency Market Research here
News-ID: 3431405 • Views: …
More Releases from Transparency Market Research
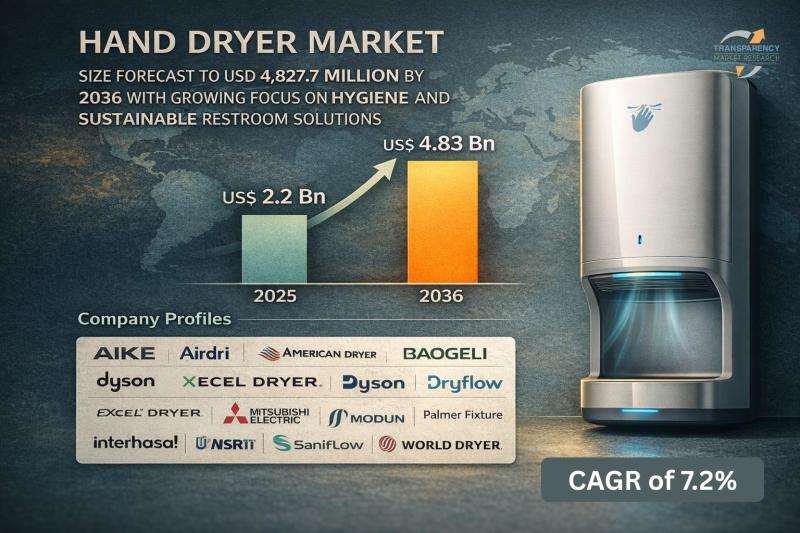
Hand Dryer Market Size Forecast to USD 4.83 Billion by 2036 with Growing Focus o …
Hand Dryer Market Outlook 2036
The global hand dryer market was valued at US$ 2.23 Billion in 2025 and is projected to reach US$ 4.83 Billion by 2036, expanding at a steady CAGR of 7.2% from 2026 to 2036. Market growth is driven by increasing emphasis on hygiene and sanitation, rising adoption in commercial infrastructure, and growing preference for eco-friendly and cost-effective hand drying solutions.
👉 Get sample market research report copy…
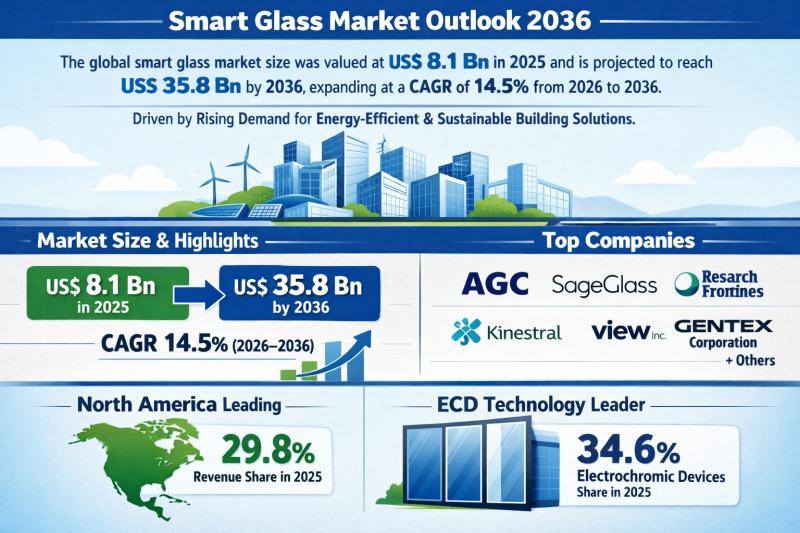
Smart Glass Market Outlook 2036: Projected to Reach USD 35.8 Billion at 14.5% CA …
The global smart glass market was valued at US$ 8.1 Bn in 2025 and is projected to surge to US$ 35.8 Bn by 2036, expanding at a robust CAGR of 14.5% from 2026 to 2036. This nearly 4.4x growth over eleven years underscores the accelerating demand for intelligent glazing solutions across commercial, residential, automotive, and infrastructure sectors.
North America emerged as the leading regional market in 2025, accounting for 29.8% of…
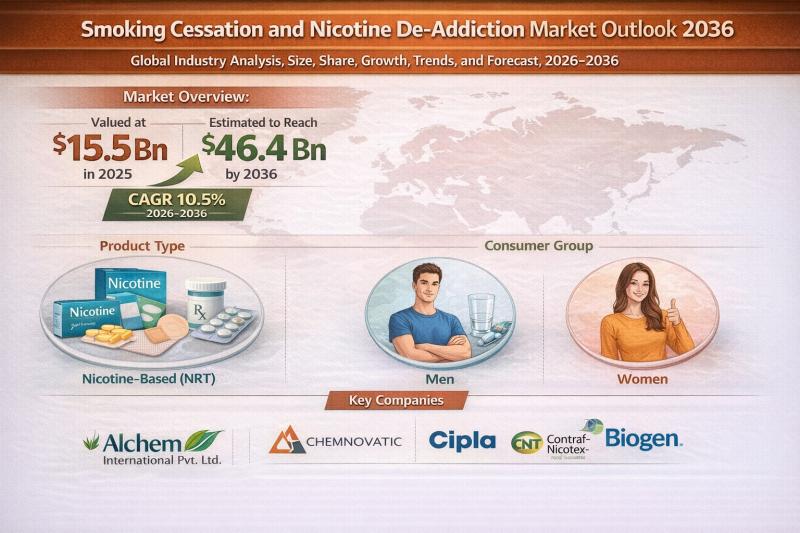
Smoking Cessation and Nicotine De-Addiction Market to Reach USD 46.4 Bn by 2036, …
The global smoking cessation and nicotine de-addiction market is witnessing strong and sustained growth, fueled by intensifying public health initiatives and rising awareness about the long-term consequences of tobacco use. Valued at USD 15.5 Bn in 2025, the market is projected to expand at a robust CAGR of 10.5% from 2026 to 2036, reaching USD 46.4 Bn by 2036.
Smoking cessation solutions encompass a wide range of products and services designed…

3D Imaging Market to be Worth USD 266 Bn by 2036 - By Component Type / By End-Us …
The global 3D imaging market is witnessing exponential growth, reflecting strong demand across healthcare, manufacturing, media, and industrial sectors. Valued at US$ 50 billion in 2025, the market is projected to reach US$ 266 billion by 2036, expanding at a robust CAGR of 18.2% from 2026 to 2036.
Get a concise overview of key insights from our Report in this sample -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=2743
This impressive trajectory highlights the rapid integration of advanced imaging…
More Releases for Regulatory
Medical Device Regulatory Affairs Market Medical Device Regulatory Affairs Marke …
"Medical Device Regulatory Affairs Market" in terms of revenue was estimated to be worth $ 6.7 billion in 2024 and is poised to reach $ 18.3 billion by 2034, growing at a CAGR of 10.8% from 2025 to 2034 according to a new report by InsightAce Analytic.
Request For Free Sample Pages:
https://www.insightaceanalytic.com/request-sample/1913
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global medical device regulatory…
Medical Device & IVD Regulatory Affairs Outsourcing Market: Navigating Regulator …
Global healthcare landscape, the Medical Device & IVD Regulatory Affairs Outsourcing Market has emerged as a critical component ensuring the safe and compliant introduction of medical devices and in-vitro diagnostic products to the market. As the industry witnesses significant shifts and challenges, here's an in-depth analysis of the current trends, dynamics, and future prospects within this market segment.
Download sample PDF copy of report: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=79264&utm_source=OpenPR_Ajay&utm_medium=OpenPR
Impact of COVID-19 on European Regulations
The outbreak of…
Regulatory Writing Market - Clear, Concise, Compliant: Redefining Regulatory Wri …
Newark, New Castle, USA - new report, titled Regulatory Writing Market The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Regulatory Writing market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Regulatory Writing market. The report offers an overview of the market, which…
Complex Regulatory Frameworks
It is challenging for new entrants to enter the FinTech industry because of its complex regulatory framework. All FinTech companies must comply with compliance requirements even before they begin operations, which increases their costs and creates a significant barrier for startups. While regulations are needed to protect consumers, a number of existing laws are slowing down the growth of many Indian FinTech companies, thereby extending their time to reach the…
South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Cr …
Presented report, South Africa Upstream Fiscal and Regulatory Report 2017 - Pending Legislation Creates Regulatory Uncertainty, presents the essential information relating to the terms which govern investment into South Africa’s upstream oil and gas sector. The report sets out in detail the contractual framework under which firms must operate in the industry, clearly defining factors affecting profitability and quantifying the state’s take from hydrocarbon production. Considering political, economic and industry…
Regulatory Affairs Outsourcing Market (Services - Regulatory Submissions, Clinic …
This research study analyzes the market for regulatory affairs outsourcing services in terms of revenue (US$ Mn). The stakeholders of this report comprises the clinical research organizations. The global regulatory affairs outsourcing market has been broadly segmented on the basis of services (Regulatory Submissions, Clinical Trial Applications and Product Registrations, Regulatory Writing and Publishing, Regulatory Consulting and Legal Representation and others regulatory affairs, and Geography (North America, Europe, Asia Pacific,…