Press release
GMP Cell Banking Services Market Size in 2023 To 2029 | WuXi AppTec, Charles River Laboratories, Eurofins Scientific, Merck KGaA, Lonza, SGS Ltd, ViruSure, Austrianova, Goodwin Biotechnology, Paragon Bioservices, BioReliance, Sartorious, BSL Bioservice, C
GMP Cell Banking Services market research delivers comprehensive insights into the current state of the market. It covers market size in terms of valuation, service volume, and offers precise predictions for the market scenario over the estimated period. This research focuses on services, applications, providers, clients, and regional segments within the GMP Cell Banking Services market. The report highlights market-driving factors, growth overview, industry volume, and market share.𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐅𝐫𝐞𝐞 𝐏𝐃𝐅 𝐒𝐚𝐦𝐩𝐥𝐞 𝐑𝐞𝐩𝐨𝐫𝐭 + 𝐃𝐞𝐭𝐚𝐢𝐥𝐞𝐝 𝐓𝐎𝐂 ➡️ https://www.reportsnreports.com/contacts/requestsample.aspx?name=6732095
𝐋𝐞𝐚𝐝𝐢𝐧𝐠 𝐩𝐥𝐚𝐲𝐞𝐫𝐬 𝐩𝐫𝐨𝐟𝐢𝐥𝐞𝐝 𝐢𝐧 𝐭𝐡𝐢𝐬 𝐫𝐞𝐩𝐨𝐫𝐭:
WuXi AppTec, Charles River Laboratories, Eurofins Scientific, Merck KGaA, Lonza, SGS Ltd, ViruSure, Austrianova, Goodwin Biotechnology, Paragon Bioservices, BioReliance, Sartorious, BSL Bioservice, Cleancells, Covance
Given the increasing demand for high-quality and regulated cell banking services, this GMP Cell Banking Services market report simplifies targeting specific services adhering to Good Manufacturing Practice (GMP) guidelines for cell banking in the global market. It aims to provide high-quality and accurate analysis, considering market predictions, regulatory compliance, technological advancements, and emerging trends in cell banking solutions.
Business stakeholders can leverage the evaluation of market dynamics to plan effective strategies and anticipate future challenges. This updated research on the global GMP Cell Banking Services market includes a micro-level analysis of service providers, clients, and key business segments during the forecasted period. The report explores opportunities, applications, services, development, innovation, and overall growth trends in GMP Cell Banking Services. Additionally, it offers strategies for companies to address the increasing demand for regulated and quality-centric cell banking services, while tracking international market trends and providing insights into service adoption in major regions.
The report incorporates primary research associated with the rapidly changing dynamics and the current scenario of the GMP Cell Banking Services industry, capturing the latest developments in banking methodologies, regulatory updates, and advancements in cell banking. Moreover, it provides insights into service functionalities, pricing structures, and dynamics of leading service providers in the GMP Cell Banking Services market, showcasing their contribution to global cell banking solutions. Region-wise segmentation of service utilization is also included. Overall, GMP Cell Banking Services market research offers comprehensive and systematic insights into global market patterns and dynamics, employing various analysis methodologies.
This report, based on historical analysis (2018-2022) and forecast calculation (2023-2029), aims to help readers to get a comprehensive understanding of global GMP Cell Banking Services market with multiple angles, which provides sufficient supports to readers strategy and decision making.
Segment by Type
- Mammalian
- Microbial
- Insect
- Yeast
- Avian
- Stem Cell
- Others
Segment by Application
- Biopharmaceutical Companies
- Contract Manufacturing Organizations
By Region
- North America
- - United States
- - Canada
➡️ 𝐈𝐧𝐪𝐮𝐢𝐫𝐞 𝐅𝐨𝐫 𝐌𝐨𝐫𝐞 𝐈𝐧𝐟𝐨𝐫𝐦𝐚𝐭𝐢𝐨𝐧 @ https://www.reportsnreports.com/contacts/inquirybeforebuy.aspx?name=6732095
The GMP Cell Banking Services report covers below items:
Chapter 1: Product Basic Information (Definition, Type and Application)
Chapter 2: Global market size, regional market size. Market Opportunities and Challenges
Chapter 3: Companies Competition Patterns
Chapter 4: Product Type Analysis
Chapter 5: Product Application Analysis
Chapter 6 to 10: Country Level Value Analysis
Chapter 11: Companies Outline
Chapter 12: Market Conclusions
Chapter 13: Research Methodology and Data Source
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release GMP Cell Banking Services Market Size in 2023 To 2029 | WuXi AppTec, Charles River Laboratories, Eurofins Scientific, Merck KGaA, Lonza, SGS Ltd, ViruSure, Austrianova, Goodwin Biotechnology, Paragon Bioservices, BioReliance, Sartorious, BSL Bioservice, C here
News-ID: 3339741 • Views: …
More Releases from ReportsnReports
DeviceCon Series 2024 - UK Edition | MarketsandMarkets
Future Forward: Redefining Healthcare with Cutting-Edge Devices
Welcome to DeviceCon Series 2024 - Where Innovation Meets Impact!
Join us on March 21-22 at Millennium Gloucester Hotel, 4-18 Harrington Gardens, London SW7 4LH for a groundbreaking convergence of knowledge, ideas, and technology. MarketsandMarkets proudly presents the DeviceCon Series, an extraordinary blend of four conferences that promise to redefine the landscape of innovation in medical and diagnostic devices.
Register Now @ https://events.marketsandmarkets.com/devicecon-series-uk-edition-2024/register
MarketsandMarkets presents…

5th Annual MarketsandMarkets Infectious Disease and Molecular Diagnostics Confer …
London, March 7, 2024 - MarketsandMarkets is thrilled to announce the eagerly awaited 5th Annual Infectious Disease and Molecular Diagnostics Conference, scheduled to take place on March 21st - 22nd, 2024, at the prestigious Millennium Gloucester Hotel, located at 4-18 Harrington Gardens, London SW7 4LH.
This conference promises to be a groundbreaking event, showcasing the latest trends and insights in diagnosis, as well as unveiling cutting-edge technologies that are revolutionizing the…

Infection Control, Sterilization & Decontamination Conference |21st - 22nd March …
MarketsandMarkets is pleased to announce its 8th Annual Infection Control, Sterilisation, and Decontamination in Healthcare Conference, which will take place March 21-22, 2024, in London, UK. With the increased risk of infection due to improper sterilisation and decontamination practices, the safety of patients and healthcare workers is of paramount importance nowadays.
Enquire Now @ https://events.marketsandmarkets.com/infection-control-sterilization-and-decontamination-conference/
This conference aims to bring together all the stakeholders to discuss the obstacles in achieving…
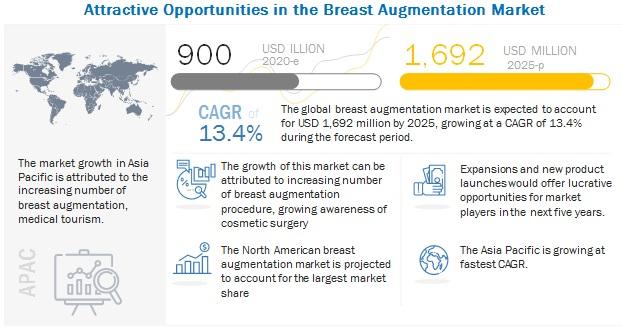
Breast Augmentation Market Key Players, Demands, Cost, Size, Procedure, Shape, S …
The global Breast Augmentation Market in terms of revenue was estimated to be worth $900 million in 2020 and is poised to reach $1,692 million by 2025, growing at a CAGR of 13.4% from 2020 to 2025. The new research study consists of an industry trend analysis of the market. The new research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
