Press release
Pharmacovigilance Market Expected to Surpass US$ 6 Billion in Revenues by 2020
Future Market Insights (FMI) delivers key insights on the global pharmacovigilance market in its upcoming outlook titled “Pharmacovigilance Market: Global Industry Analysis and Opportunity Assessment, 2015 - 2020”. In terms of revenue, the global pharmacovigilance market is estimated to expand at a healthy CAGR of 14.2% through 2020. The global pharmacovigilance market was valued at US$ 2,759.1 Mn in 2014 and is expected to reach US$ 6,104.1 Mn in 2020, expanding at a CAGR of 14.2% from 2015 to 2020.The market is segmented based on phase of drug development, type of reporting methods and type of service providers.
Based on phase of drug development, the market has been segmented into preclinical studies, phase I clinical trial, phase II clinical trial, phase III clinical trial and phase IV clinical trial or post-marketing surveillance.
Based on type of reporting methods, the market has been segmented into spontaneous reporting, intensified ADR reporting, targeted spontaneous reporting, cohort event monitoring and EHR mining.
On the basis of service providers, the market has been segmented into in-house and contract outsourcing. As the number of chemical entities has been growing in the global pharmaceuticals market, pharmacovigilance is increasingly become a mandate for drug manufacturers. Considering the change in regulations, phase III and phase IV studies are being increasingly conducted to monitor the long-term safety outcomes of pharmaceuticals and biological products. Implementation of active pharmacovigilance activities assists in the execution of long-term plans, such as in bringing improvement in patient outcome and minimisation of health care associated costs, particularly related to Adverse Drug Reactions (ADRs), and in the prevention of drug hazards.
To provide deeper insights into the pharmacovigilance market, the report has also been segmented by phase of drug development, type of reporting method and type of service provider.
Browse the full "Pharmacovigilance Market: Global Industry Analysis and Opportunity Assessment, 2015 - 2020" market research report at http://www.futuremarketinsights.com/reports/pharmacovigilance-market
By phase of drug development, the clinical trial phase III segment is projected to expand at a CAGR of 15.5% in the global pharmacovigilance market by phase of drug development by 2020 end in terms of value. Evaluation of real-time effectiveness of the drug and availability of a facility to conduct trials in state-of-the-art settings, thus complementing the outcomes of premarketing randomised control trials, are the prime benefits availed from pharmacovigilance in phase IV trials. It has also been reported that approximately 57% of the global pharmaceutical companies outsource post marketing operations to CROs in order to avoid the high operational cost associated with technological infrastructure and hiring skilled staff.
The primary barriers in the market include high risk associated with securing data in case of pharmacovigilance outsourcing and lack of availability of skilled workforce specialising in drug monitoring.
The primary trend in the pharmacovigilance market is an increase in dependence on third party services. Other trends include harnessing shorter turnaround times for faster market capitalisation and requirement of highly skilled personnel for monitoring side effects.
Some of the key players identified in the global pharmacovigilance market report include: Accenture plc, Bristol-Myers Squibb, Clinquest Group B.V., Cognizant Technology Solutions, Covance, Inc., F. Hoffmann-La Roche Ltd., GlaxoSmithKline plc (GSK), ICON plc, iGATE Corporation, iMEDGlobal Corporation, inVentiv Health, Inc., Novartis International AG, PAREXEL International Corporation, Pfizer, Inc., Pharmaceutical Product Development, LLC. (PPD), PRA Health Sciences, Inc., Quintiles Transnational Holdings, Inc., Sanofi S.A., Synowledge LLC and Wipro Limited. These companies specialise in pharmacovigilance services, and focus on market consolidation initiatives and analyses of their specific strengths, weaknesses, opportunities and threats to strengthen their position in the market.
Request Free Report Sample@ http://www.futuremarketinsights.com/reports/sample/rep-gb-1107
The report has been concluded with strategic recommendations for players already present in the market and new players planning to enter the market, which could help them in the near future.
This report assesses factors driving growth of each segment of the market and presents analysis and key insights on the potential of the pharmacovigilance market as per region-specific trends. North America accounted for majority of the pharmacovigilance market revenue in 2014. However, incidences of increasing consumption of drugs and rise in cases of adverse drug reactions are expected to drive growth of the market in Asia Pacific and Latin American regions.
Future Market Insights (FMI) is a leading market intelligence and consulting firm. We deliver syndicated research reports, custom research reports and consulting services, which are personalized in nature.
616 Corporate Way, Suite 2-9018,
Valley Cottage, NY 10989,
United States
T: +1-347-918-3531
F: +1-845-579-5705
Email: sales@futuremarketinsights.com
Website: www.futuremarketinsights.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Pharmacovigilance Market Expected to Surpass US$ 6 Billion in Revenues by 2020 here
News-ID: 325252 • Views: …
More Releases from Future Market Insights
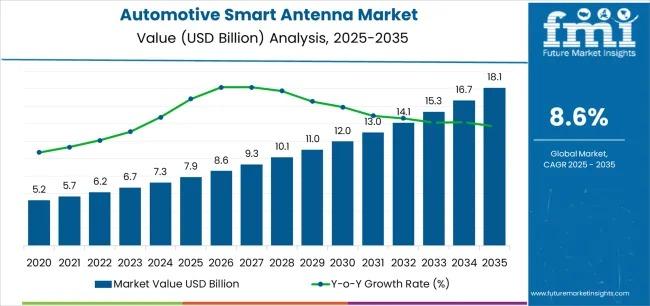
Global Automotive Smart Antenna Market to Reach USD 18.1 Billion by 2035, Driven …
The global automotive smart antenna market is projected to grow from USD 7.9 billion in 2025 to USD 18.1 billion by 2035, registering a robust compound annual growth rate (CAGR) of 8.6% during the forecast period. This expansion reflects the accelerating transformation of the automotive sector toward connected mobility, advanced communication infrastructure, and data-driven vehicle ecosystems. Increasing adoption of 5G-enabled connectivity, advanced driver assistance systems (ADAS), and vehicle-to-everything (V2X) communication…
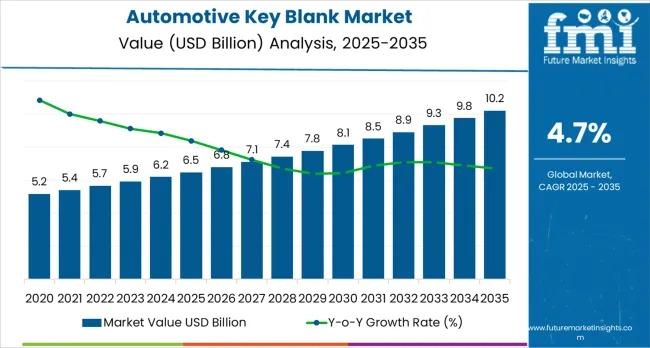
Automotive Key Blank Market to Reach USD 10.2 Billion by 2035 as Smart Key Integ …
The global automotive key blank market is projected to grow from USD 6.5 billion in 2025 to USD 10.2 billion by 2035, reflecting a steady compound annual growth rate (CAGR) of 4.7%. This expansion is being driven by rising vehicle production, increasing adoption of smart and transponder-based security systems, and sustained replacement demand across global automotive fleets. As vehicle access systems evolve toward integrated electronic security frameworks, automotive key blanks…
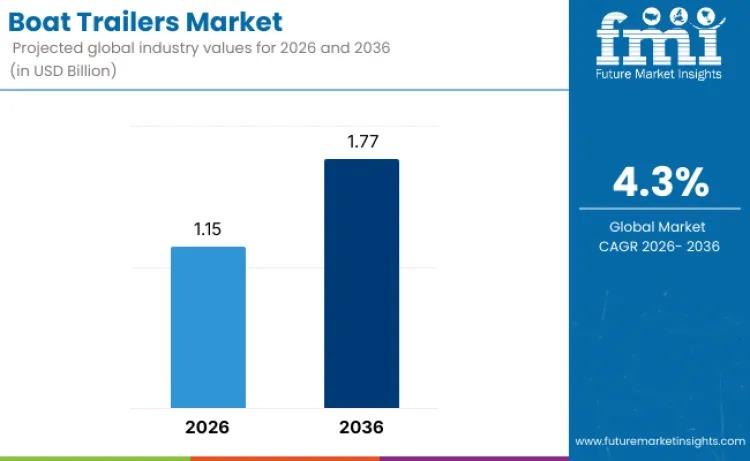
Global Boat Trailers Market to Reach USD 1.8 Billion by 2036 as Recreational Boa …
The global boat trailers market is poised for steady and sustained growth, with industry valuation projected to increase from USD 1.1 billion in 2026 to USD 1.8 billion by 2036, expanding at a compound annual growth rate (CAGR) of 4.3%. This expansion reflects rising global participation in recreational boating, increasing disposable incomes, and expanding investments in marine tourism infrastructure. As water-based leisure activities gain popularity across developed and emerging economies,…
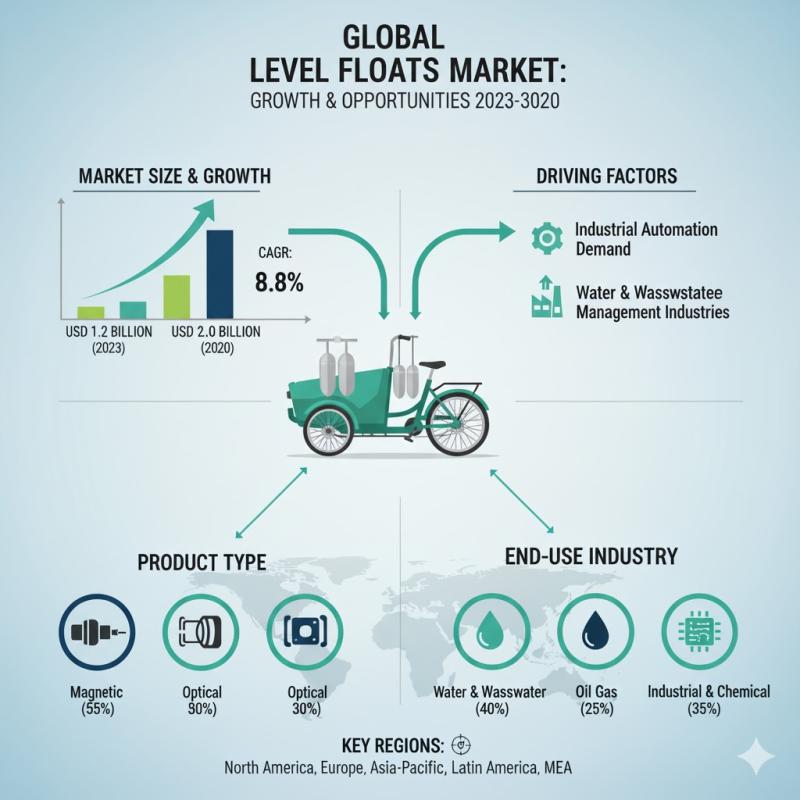
Level Floats Market to Reach USD 461.7 Million by 2035 as Automotive Electrifica …
The global level floats market is projected to grow from USD 311.9 million in 2025 to USD 461.7 million by 2035, registering a steady compound annual growth rate (CAGR) of 4.0% over the forecast period. This expansion reflects rising demand for precision fuel measurement systems across automotive, industrial, and energy applications, supported by increasing vehicle production, evolving fuel management technologies, and stricter regulatory standards focused on efficiency and emissions compliance.
Level…
More Releases for Pharmacovigilance
Top Pharmacovigilance Companies Analysis By 2031
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
Download PDF Copy @ https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpenPR&utm_medium=10379
The List of Companies
• #Accentures
• Bristol-Myers Squibb Company
• Linical Accelovance
• Cognizant
• Covance Inc.
• F. Hoffmann-La Roche Ltd.
• GlaxoSmithKline plc.
• ICON plc
• Capgemini (IGATE Corporation)
Clinical…
Pharmacovigilance - Scope and Research Methodology
The Pharmacovigilance Market is expected to register a CAGR of 6.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The Pharmacovigilance Market report covers analysis by Clinical Trial Phase (Pre-Clinical, Phase I, Phase II, Phase III, and Phase IV), Service Provider (In-House and Contract Outsourcing), Type of Method (Spontaneous Reporting, Intensified ADR Reporting, Targeted Spontaneous Reporting, Cohort Event…
Pharmacovigilance World 2025 Conference & Expo
We are delighted to welcome you to the Pharmacovigilance World 2025, and we are confident that your active participation will contribute to the advancement of drug safety practices. Together, let us strive towards a safer and more vigilant healthcare system that prioritizes patient well-being and ensures the continued benefit of medications worldwide.
As medical science advances, so does our understanding of drug safety and the need for vigilance when it comes…
Top Factor Driving Pharmacovigilance Market Growth in 2025: Research And Develop …
How Are the key drivers contributing to the expansion of the pharmacovigilance market?
The escalation in research and development undertakings stimulates growth in the pharmacovigilance market. Pharmaceutical organizations can create novel and superior drugs through enhanced safety profiles by allocating resources to R&D. The intensive testing in preclinical and clinical stages during the drug development protocol allows early recognition of potential safety issues, paving the way for adequate risk reduction approaches.…
Monitoring Medication Safety with Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem. Pharmacovigilance plays a significant role in pharmaceutical and biotechnological sectors in designing of drugs and their interactions. The pharmacovigilance involves collecting information from healthcare providers and patients to know about the hazards associated with medications.
Download Sample PDF at: https://www.theinsightpartners.com/sample/TIPRE00003127?utm_source=OpnePR&utm_medium=10776
Increasing cases of adverse drug reactions…
Pharmacovigilance Market Opportunity Analysis by 2028
Pharmacovigilance Market: Introduction
According to the report, the global pharmacovigilance market was valued at US$ 6.1 Bn in 2020 and is projected to expand at a CAGR of 8.8% from 2021 to 2028. Pharmacovigilance activities are defined as science used for detection, assessment, understanding, and prevention of adverse effects of drugs and vaccines. Drugs and vaccines go through rigorous testing in the clinical trials to check their safety and efficacy before…
