Press release
Clinical Trials Market's Remarkable 4.41% CAGR Journey to US$ 177.7 Billion by 2033 | FMI Inc.
By the end of 2023, the global Clinical Trials Market is anticipated to be worth US$ 115.4 billion, and it will grow at a CAGR of 4.41% to reach US$ 177.7 billion by the year 2033. Industry sponsors are in the lead with a predicted market share of roughly 54.7% in 2023, according to a recent analysis by Future Market Insights, inside the global Clinical Trials market.Clinical trials play a crucial role in advancing the understanding of rare diseases and developing potential therapies. These trials are designed to evaluate the safety and efficacy of new drugs, therapies, or interventions in a controlled and scientific manner. They typically involve testing the investigational product on a small number of patients with the rare disease under strict protocols.
Secure Your Copy and Stay Ahead in the Dynamic Rare Disease Clinical Trials Market @
https://www.futuremarketinsights.com/reports/sample/rep-gb-16889
According to FMI, the rare disease clinical trials market size is projected to be valued at US$ 12,566.14 million in 2023 and is expected to rise to US$ 31,715.25 million by 2033. The sales of rare disease clinical trials are expected to expand at a significant CAGR of 9.7% during the forecast period. The report states that increasing clinical trials for rare diseases have grown significantly with numerous biopharmaceutical companies focusing on rare diseases.
One of the key drivers of market growth is the Orphan Drug Act. It provides incentives to pharmaceutical companies to develop treatments for rare diseases. Additionally, the rise in funding for rare disease research from both private and public sources has also contributed to the growth of the market. Another key trend in the market is the use of innovative technologies such as gene therapy and precision medicine in rare disease clinical trials. These technologies offer the potential for more targeted and effective treatments for rare diseases.
Key Takeaways from the Rare Disease Clinical Trails Market
-In terms of phase, Phase III is expected to have a high CAGR of 10.3% during the forecast period. The high proportion of Phase III trials is due to the fact that they are expensive and involve subsequent subjects. In phase III trials, long-term safety studies are conducted for registration and post-marketing commitments.
-The high prevalence of rare diseases and the presence of a robust healthcare system for diagnosis and treatment are anticipated to expand North America to 49.3%.
-Pharmaceutical and biopharmaceutical companies recorded a strong revenue share of 58.5% in 2022. Pharmaceutical companies are actively involved in rare disease clinical trials through collaboration with other companies.
-The Asia Pacific region is expected to hold a swift rate of 10.6%. The region's expansion can be attributed to government initiatives to assist orphan disease patients. For example, the Indian government directed national and state governments in July 2022, to ensure the effective implementation of health policies developed to treat patients suffering from orphan diseases.
-During the forecast period, the nonprofit organization segment is expected to secure a CAGR of 9.9%. Non-profit and other public organizations are actively involved in funding rare disease clinical research to support the development of potential treatments for rare diseases.
Competitive Landscape:
The market for rare disease treatment is competitive, with several leading players. A few key players are dominating the market in terms of market share. Amgen Inc., AstraZeneca (Alexion Pharmaceuticals Inc.), Bristol-Myers Squibb Company, Biomarin Pharmaceuticals, and Bayer AG are among the companies currently dominating the market. Market participants readily implement a variety of initiatives, such as mergers and acquisitions and product launches, to strengthen their market position.
Latest Developments:
-ProtalixBiotherapeutics Inc. and Chiesi Global Rare Diseases resubmitted the Biologics License Application (BLA) to the United States Food and Drug Administration (FDA) in November 2022. The license was submitted for PRX-102 (pegunigalsidasealfa) which is used for the treatment of adult patients with Fabry disease.
-The National Institutes of Health, the United States Food and Drug Administration, ten pharmaceutical companies, and five non-profit organizations joined forces in October 2022. The collaboration took place to accelerate the development of gene therapies for people suffering from rare diseases.
Gain Valuable Insights Through Our Cutting-Edge Methodology Today! @
https://www.futuremarketinsights.com/request-report-methodology/rep-gb-16889
Key Segments Covered
Therapeutic Area Outlook:
-Oncology
-Cardiovascular Disorders
-Neurological Disorders
-Infectious Disease
-Genetic Disorders
-Autoimmune And Inflammation
-Hematologic Disorders
-Musculoskeletal Disorders
-Others
Phase Outlook:
-Phase I
-Phase II
-Phase III
-Phase IV
Sponsor Outlook:
-Pharmaceutical & Biopharmaceutical Companies
-Non-profit Organizations
-Others
Author
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
Contact Us:
Nandini Singh Sawlani
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Get Full Report for Complete Data: @
https://www.futuremarketinsights.com/reports/clinical-trials-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5,000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Clinical Trials Market's Remarkable 4.41% CAGR Journey to US$ 177.7 Billion by 2033 | FMI Inc. here
News-ID: 3192618 • Views: …
More Releases from Future Market Insights Inc.

Olive Phenolic Complexes for Metabolic Health Market to Surpass USD 2,420 Millio …
The global olive phenolic complexes for metabolic health market is set for robust expansion, rising from USD 710 million in 2026 to USD 2,420 million by 2036, reflecting a strong compound annual growth rate (CAGR) of 12.4%. Growth momentum is fueled by rising global awareness of metabolic health and increasing adoption of plant-based bioactive compounds in preventive nutrition.
Olive phenolic complexes, rich in bioactive compounds such as oleuropein and hydroxytyrosol, are…
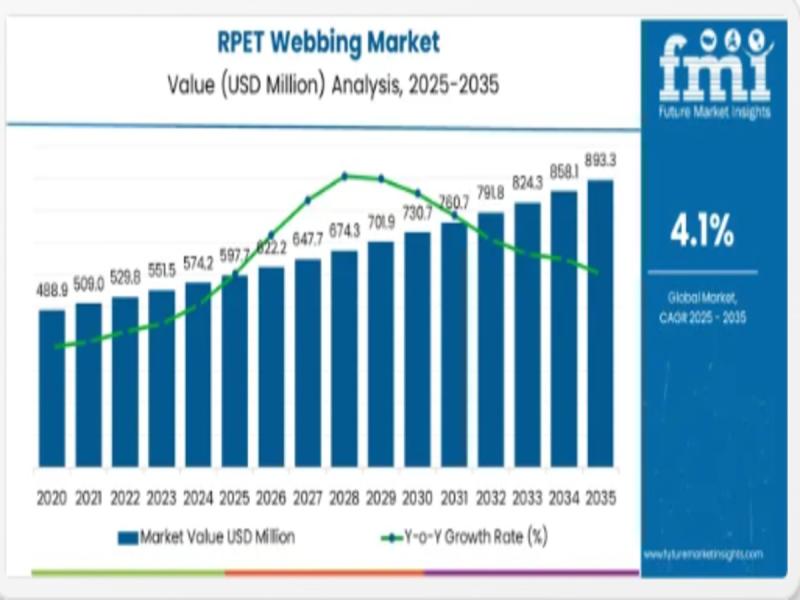
Global RPET Webbing Market Set to Surge to Nearly USD 893 Million by 2035 as Sus …
The global Recycled Polyethylene Terephthalate (RPET) webbing market is projected to expand from an estimated USD 597.7 million in 2025 to approximately USD 893.2 million by 2035, reflecting robust momentum in recycled materials adoption across key industrial and consumer sectors and underscoring sustainability as a core manufacturing imperative. The market is expected to grow at a compound annual growth rate (CAGR) of 4.1 % during this forecast period, driven by…
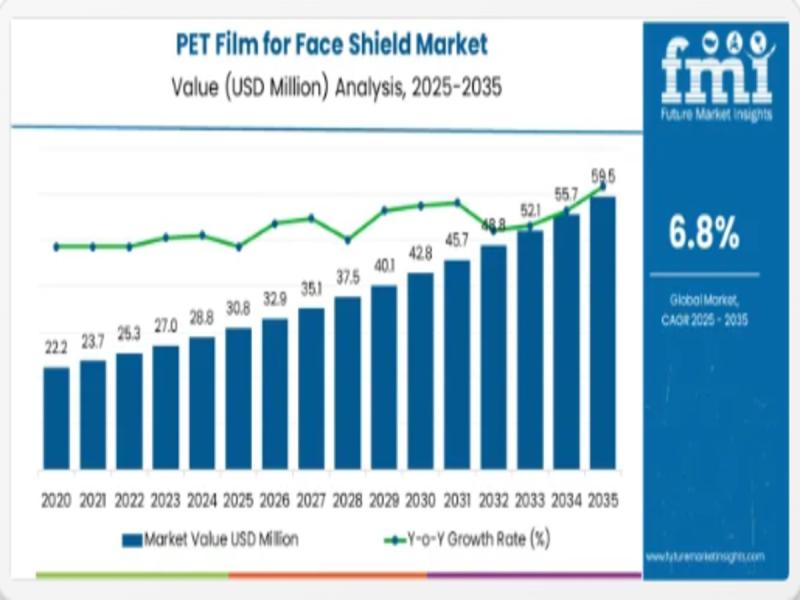
PET Film for Face Shield Market - Strategic Growth, Innovation & Forecasted Surg …
The global PET film for face shield market is set to expand from an estimated USD 30.8 million in 2025 to approximately USD 59.5 million by 2035, representing strong demand for high-clarity protective materials across healthcare and industrial safety sectors with a compound annual growth rate (CAGR) of 6.8% over the decade. This growth underscores the rising prioritization of personal protective equipment (PPE) globally, especially where transparent barrier films combine…

Middle East and North Africa Frozen Food Market Poised for Steady Growth Through …
Middle East and North Africa Frozen Food Market
The Middle East and North Africa (MENA) frozen food market is set for consistent and resilient growth over the next decade, supported by shifting consumer lifestyles, expanding modern retail infrastructure, and rising demand for long-shelf-life food solutions suited to the region's climate. The market is estimated to be valued at USD 1.4 billion in 2025 and is projected to reach USD 1.8 billion…
More Releases for Clinical
Miami Clinical Research Sets the Standard for Clinical Trials
Miami Clinical Research, a frontrunner in the world of clinical trials and medical research, has emerged as the prime choice for global corporate pharmaceutical giants. With a deep understanding of the complexities of medical studies, the organization champions the crucial role of research in the evolution of transformative therapeutic interventions.
Miami, FL - Renowned as a first-rate center for professional medical exploration, Miami Clinical Research [https://miamiclinicalresearch.com] boasts state-of-the-art facilities, advanced technologies,…
E-Clinical Solutions Market: Revolutionizing Healthcare and Clinical Trials
Introduction
The e-Clinical solutions market has become a pivotal component of the healthcare and pharmaceutical industries. E-Clinical solutions refer to a set of software, tools, and platforms designed to streamline clinical trials and healthcare management. These solutions include electronic data capture (EDC), clinical trial management systems (CTMS), laboratory information management systems (LIMS), and other integrated tools that improve the efficiency, accuracy, and speed of clinical trials and healthcare services. The primary…
E-Clinical Solutions Market: Revolutionizing Clinical Trials
The e-clinical solutions market has experienced significant growth in recent years, driven by the increasing complexity of clinical trials and the need for efficient, accurate, and compliant data management. E-clinical solutions provide a comprehensive suite of tools and technologies to streamline clinical trial processes, accelerate drug development, and improve patient outcomes.
Market Size and Growth
The global e-clinical solutions market is estimated to be worth billions of dollars, with a significant portion…
Clinical Trials Management System Market Optimizing Clinical Trials: The Crucial …
Clinical Trials Management System Market to reach over USD 5.06 billion by the year 2031- Exclusive Report by InsightAce Analytic
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the "Global Clinical Trials Management System Market Size, Share & Trends Analysis Report By Solution Type (Enterprise and Site based), By Delivery Mode (Web & Cloud-based, On-premise), By Component (Software, Services), By End-user (Pharmaceutical and Biotechnology Firms, Medical…
Clinical Research and Clinical Trials Summit
Clinical Research 2019 has been designed in an interdisciplinary manner with a multitude of tracks to choose from every segment and provides you with a unique opportunity to meet up with peers from both industry and academia and establish a scientific network between them. We cordially invite all concerned people to come join us at our event and make it successful by your participation.
This is the premier interdisciplinary forum for…
E-Clinical Trial Solutions Market To Accelerating Clinical Development Technolog …
The study of the "Global e-Clinical Trial Solutions Market" provides the market size information and market trends along with the factors and parameters impacting it in both short and long term. The study ensures a 360° view, bringing out the complete key insights of the industry.
The Global e-Clinical Trial Solutions Market Research Report Forecast 2017-2021 is a valuable source of insightful data for business strategists. It provides the e-Clinical…
