Press release
Quality Preserved: Exploring the GMP Storage Market's Evolution in 2033 - Safeguarding Pharmaceuticals, Biologics, and Medical Supplies with State-of-the-Art Storage Solutions
At the end of 2021, the global GMP storage market generated around US$ 5.7 billion in revenue. By the end of 2032, the market is expected to have grown by 5.6% at a CAGR of value. By the end of 2032, Persistence Market Research projects that the market for GMP storage devices will be worth US$ 8.3 Bn. Global sales of GMP storage products and services represent roughly 4.7% of the market's total revenue, which was estimated to be worth about US$119.98 Bn at the end of 2021.Request for Free Sample Copy@ https://www.persistencemarketresearch.com/samples/33113
A historic CAGR of 4.4% was seen in the last nine years, from 2012 to 2021, on the global GMP storage market. The development of large molecule biological products that need to be stored chilled or frozen has been spurred by the current emphasis on vaccine manufacture. The global market is positively impacted by these new items, but temperature-controlled GMP storage is essential.
The cost of building and maintaining temperature-controlled GMP storage facilities is the first consideration. In addition to refrigeration equipment, such facilities require significant security, building management/temperature monitoring, inventory management, and backup power systems. For the upkeep and operation of the equipment as well as regulatory compliance, they also require qualified personnel.
Get Full Report Now @ https://www.persistencemarketresearch.com/checkout/33113
Companies
ThermoGenesis Holdings, Inc.
ThermoFisher Scientific Inc.
BioLife Solutions, Inc.
Danaher(Cytiva)
MEDIPOST
REMI GROUP
Eurofins Scientific
Eppendorf
Intertek Group Plc
Arctiko
Bioline Technologies
Hindustan Apparatus Mfg. Co.
P L Tandon & Co.
Stericox India Private Limited
SY-LAB Geräte GmbH
BioConvergence LLC d/b/a Singota Solutions
PHC Holdings Corporation
Pace Life Sciences
Haier Biomedical
Helmer Scientific Inc.
Competitive Landscape
To strengthen their product lines around the globe, leading manufacturers are developing technologically-advanced products. Similarly, several major competitors in the GMP storage industry have engaged in consolidation activities such as mergers & acquisitions. Another significant approach noticed in the industry is the expansion of corporate collaborations to boost their GMP storage services.
BioLife Solutions, Inc. acquired Global Cooling, Inc. in May 2021, which has a portfolio of freezers ranging in size from portable units to stationary upright freezers to suit a wide range of applications.
BioLife Solutions debuted a new high-capacity, controlled-rate freezer line in April 2021, with the first shipment going to a leading cell treatment provider.
Request For Customization @ https://www.persistencemarketresearch.com/request-customization/33113
Key Segments Covered in GMP Storage Industry Research
GMP Storage Market by Product & Service:
GMP Storage Products
Refrigerators and Freezers
Cryogenic Storage
GMP Storage Services
GMP Storage Market by Application:
Cell & Gene Therapy
Cell Banking
Biologics
Small Molecules
Others
GMP Storage Market by End User:
Biopharmaceutical Companies
Contract Manufacturing Organizations
Contract Research Organizations
Research & Academic Institutes
GMP Storage Market by Region:
North America GMP Storage Market
Latin America GMP Storage Market
Europe GMP Storage Market
South Asia GMP Storage Market
East Asia GMP Storage Market
Oceania GMP Storage Market
The Middle East & Africa GMP Storage Market
Persistence market research
Address - 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. - +1-646-568-7751
USA-Canada Toll-free - +1 800-961-0353
Sales - sales@persistencemarketresearch.com
About us: -
Persistence Market Research is a U.S.-based full-service market intelligence firm specializing in syndicated research, custom research, and consulting services. Persistence Market Research boasts market research expertise across the Healthcare, Chemicals and Materials, Technology and Media, Energy and Mining, Food and Beverages, Semiconductors and Electronics, Consumer Goods, and Shipping and Transportation industries. The company draws from its multi-disciplinary capabilities and high-pedigree team of analysts to share data that precisely corresponds to clients' business needs.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Quality Preserved: Exploring the GMP Storage Market's Evolution in 2033 - Safeguarding Pharmaceuticals, Biologics, and Medical Supplies with State-of-the-Art Storage Solutions here
News-ID: 3189290 • Views: …
More Releases from Persistence Market Research
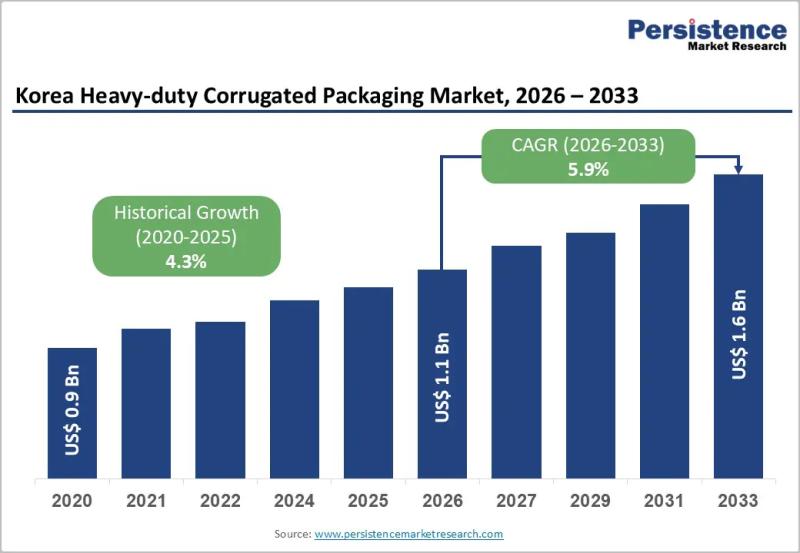
Korea Heavy-duty Corrugated Packaging Market to Reach US$1.6 Billion by 2033 - P …
The Korea heavy-duty corrugated packaging market plays a critical role in supporting industrial logistics, bulk transportation, and export-driven manufacturing. Heavy-duty corrugated packaging is widely used for shipping machinery, automotive components, electronics, chemicals, and large industrial goods that require superior strength and structural integrity. Unlike conventional corrugated boxes, heavy-duty variants are engineered with multi-wall boards, reinforced liners, and customized structural designs to withstand high load capacity, stacking pressure, and long-distance transportation.…

Textile Flooring Market Set for Steady Growth as Demand for Sustainable and Styl …
The global textile flooring market is entering a phase of stable expansion, supported by rising construction activity, increasing consumer focus on interior aesthetics, and growing demand for eco-friendly flooring solutions. According to industry estimates, the global textile flooring market size is likely to be valued at US$11.1 billion in 2026 and is projected to reach US$16.5 billion by 2033, expanding at a CAGR of 5.8% between 2026 and 2033. This…

Power System Simulator Market Size to Reach US$ 2.6 Billion by 2033 - Persistenc …
The power system simulator market is gaining strategic importance as global energy systems transition toward digitalization, decentralization, and decarbonization. Power system simulators are advanced software and hardware platforms used by utilities, grid operators, engineering firms, and research institutions to model, analyze, and optimize electrical power networks. These simulators enable real time grid analysis, contingency planning, load flow studies, fault analysis, stability assessment, and operator training. As electricity networks become more…

Yoga and Meditation Products Market Set for Robust Growth, Projected to Reach US …
The global wellness industry is undergoing a major transformation as consumers increasingly prioritize mental health, mindfulness, and preventive self-care. Within this evolving landscape, the yoga and meditation products market has emerged as a fast-growing segment, encompassing everything from yoga mats and apparel to meditation cushions, smart devices, and digital-enabled accessories. According to industry estimates, the global yoga meditation products market is projected to be valued at US$ 8.3 billion in…
More Releases for GMP
Creative Peptides Released GMP Synthesis Service
Located in Shirley, New York, the world’s leading peptide supplier Creative Peptides announced the launch of its GMP synthesis (https://www.creative-peptides.com/services/custom-gmp-peptide-synthesis-services.html ) business on August 29, 2018. Now this company is focused on the development and GMP manufacturing of pharmaceutical grade peptides.
As the demand of pharmaceutical market continues to grow, more and more pharmas and research institutions choose the CMO and CRO models to expand their businesses, which is more…
Diapharm implements European GMP guidelines in China
Münster (DE), London (UK), Ningbo (CN), 20 December 2013 – Pharmaceutical service provider Diapharm (diapharm.com) is increasing its business activities in China: Diapharm has now implemented a “European” quality management system for Neptune Pharma Ltd (www.neptunepharma.com) in their Joint Venture Partner’s factory in Ningbo, Zhejiang Province. And it has done so successfully: The veterinary medicinal product Trident 500mg/g Powder for Suspension for Fish Treatment (www.trident-50.com), is manufactured onsite under EU…
ECA Foundation releases free GMP WebApp
The ECA Foundation has been providing advanced training and information services in the pharmaceutical industry and especially with regard to pharmaceutical Quality Assurance and GMP compliance for more than 10 years. Now the organisation took advantage of its extensive experience to develop a further free of charge service – the new GMP WebApp.
This new GMP WebApp runs on all smartphones and tablet PCs (Apple and Android platforms) and allows users…
GMP Friction Products Awarded ISO 9001:2008
Internationally Recognized Certification Measures Consistency in Process, Procedure and Quality Performance in Manufacture of Friction Materials
AKRON, OH (March 23, 2011) -- GMP Friction Products, a world leader manufacturing powdered metal friction products for clutch plates and brake pads, recently received certification for ISO 9001:2008.
“ISO 9001:2008 signifies we have taken the extra measure of documenting the policies and standards to ensure consistent compliance with our manufacturing processes,” said Jerry Lynch,…
GMP MANUAL Volume 2 - Validation Procedures by Maas & Peither AG – GMP Publish …
GMP Publishing is launching its new GMP MANUAL Volume 2 – Validation Procedures.
The compendium on validation procedures was written by Dr. Doris Borchert, Dr. Peter Bosshard, Dr. Ralph Gomez, Dr. Michael Hiob, Dr. Christine Oechslein, Max Lazar, Ulrike Reuter, Michael Schulte, Uwe Schwarzat – all international experts and key opinion leaders. They share their detailed understanding of the various aspects of the validation process in clear and comprehensive style…
blue inspection body celebrates 50 GMP audits
Münster (Germany), 20 November 2009. Two years after founding the company and just 18 months after gaining the accreditation blue inspection body GmbH announced today the successful execution of its 50th GMP audit. Further audit trips to China, India, Israel and various European countries have been scheduled already, meaning that in the first quarter 2010 the 75th audit is targeted to be completed. Blue, as a privately organised inspection body,…
