Press release
Werum IT Solutions introduces new service for the cost-effective, high-quality and large-scale MBR creation
Werum IT Solutions introduces new service for the cost-effective, high-quality and large-scale MBR creation“Design as a Service” offers the customer-specific creation of MBRs and the efficient operation of Werum’s PAS-X manufacturing execution system
Lüneburg, Germany, September 23, 2015 – Development of new drugs, process harmonization and standardization or efficient operation of manufacturing execution systems (MES) – pharmaceutical and biopharmaceutical companies face major challenges to remain competitive. In this regard, electronic master batch records (MBRs) are an essential factor.
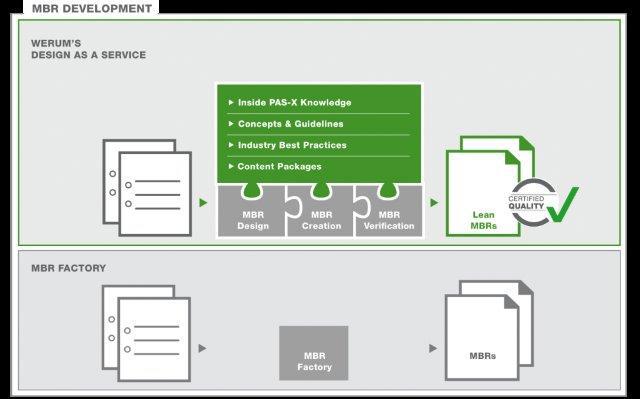
Werum IT Solutions now offers its customers a new service: “Design as a Service” – a complete solution for the cost-effective creation of lean, high-quality and streamlined MBRs. The package comprises pre-configured PAS-X Content Packages with industry-specific content based on best practices, ready-to-use concepts and guidelines for the efficient creation and optimization of MBRs and profound, first-hand PAS-X knowledge. As the company who developed PAS-X, Werum is able to provide an “end-to-end” MES solution that meets all customer requirements in terms of software, delivery and services.
Through “Design as a Service”, Werum does not only support the cost-effective high-quality creation of MBRs for new MES installations but also supports customers who already operate an MES and rather focus on process harmonization, PAS-X upgrades or the MBR creation for new products (ad-hoc service). As PAS-X is Werum’s proprietary software product, Werum can also consider future PAS-X functions when creating MBRs. “Design as a Service” addresses all phases of MBR development, such as MBR specification, MBR creation and finally MBR verification. The resulting MBRs are lean, efficient and optimized to meet the customer requirements in regard to “Review by Exception”.
“Our ‘Design as a Service’ offering allows us to provide our pharmaceutical and biopharmaceutical customers with much more than the mass conversion of paper-based recipes into digital copies which is usually referred to as MBR factory,” says Martin Gent, Consultant, Werum IT Solutions GmbH. “Our approach starts at an earlier stage and we reflect about the manufacturing processes together with our customers. Even during the MBR specification phase we consider the entire spectrum of manufacturing procedures, system environment and review processes. In combination with the profound process know-how of our staff we ensure optimized high-quality MBRs from the very beginning – an important prerequisite for the efficient operation of our PAS-X MES. Customers who want to save costs by commissioning the mere creation of MBRs often face extra costs when they need to revise their MBRs.”
Werum IT Solutions is the world’s leading supplier of manufacturing execution systems (MES) and manufacturing IT solutions for the pharmaceutical and biopharmaceutical industries. Its out-of-the-box PAS-X software product is run by 17 of the world’s top 30 pharmaceutical and biotech companies but also by many mid-sized manufacturers. Werum’s manufacturing IT solutions help pharma manufacturers to increase efficiency, improve productivity, and meet regulatory requirements. Founded in 1969, the IT company employs about 420 people at its headquarters in Lüneburg, Germany, and at ten other locations in Europe, America and Asia.
Werum is part of Medipak Systems, the Pharma Systems business area of the international Körber technology group. Körber unites worldwide nearly 12,000 professionals in industry-leading companies, achieving annual earnings of more than 2.3 billion Euros. As a Medipak Systems company, Werum provides integrated IT solutions for all phases of pharmaceutical and biopharmaceutical production – including process development, commercial production, and packaging as well as track & trace serialization.
For more information take a look at our website www.werum.com.
Dirk Ebbecke
Director Corporate Communications
Werum IT Solutions GmbH
Wulf-Werum-Str. 3
21337 Lüneburg, Germany
Tel. +49 4131 8900-689
Fax +49 4131 8900-20
dirk.ebbecke@werum.com
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Werum IT Solutions introduces new service for the cost-effective, high-quality and large-scale MBR creation here
News-ID: 316772 • Views: …
More Releases from Werum IT Solutions GmbH
Boosting paperless pharma manufacturing in India: strategic partnership of Werum …
Werum and Sarla to partner for delivering MES projects in India / Multinationals and local pharma companies will benefit from the number one global pharma MES solution and smooth integration of the automation level
Lüneburg, Germany, September 15, 2015 – Werum IT Solutions, the world’s leading supplier of manufacturing execution systems (MES) and manufacturing IT solutions for the pharmaceutical and biopharmaceutical industries, and Sarla Technologies jointly announced a memorandum of understanding…

Leading mid-sized contract manufacturer Losan Pharma introduces Werum’s PAS-X …
Leading mid-sized contract manufacturer Losan Pharma introduces Werum’s PAS-X for production of solid dosage forms
Implementation in the main plant in Neuenburg am Rhein, Germany / Full-scope MES including warehouse management / Fully integrated Track & Trace
Lüneburg, Germany, August 11, 2015 – Losan Pharma is going to introduce the latest version of Werum’s PAS-X MES in their main plant in Neuenburg am Rhein, Germany. The medium-sized company specializes in contract research…
More Releases for MBR
Membrane Bioreactor (MBR) Market Size 2024 to 2031.
Market Overview and Report Coverage
A Membrane Bioreactor (MBR) is an advanced wastewater treatment technology that combines membrane filtration with biological treatment processes. It provides high-quality treated water by removing suspended solids, pathogens, and organic matter.
The current outlook for the Membrane Bioreactor (MBR) Market is positive, with a steady growth expected at a CAGR of 3.20% during the forecasted period. This growth can be attributed to the increasing demand…
Membrane Bioreactor Market Analysis and Forecast to 2033: By Configuration (Subm …
Market Definition
The Membrane Bioreactor (MBR) Market stands at the forefront of wastewater treatment technology, offering efficient and sustainable solutions for water purification. This market encompasses the production, development, and distribution of membrane bioreactor systems-an advanced wastewater treatment technology that combines biological treatment and membrane filtration. MBR systems contribute to the removal of contaminants, pathogens, and pollutants from wastewater, providing a versatile and compact solution for various applications, including municipal, industrial,…
Membrane Bioreactor (MBR) Systems Market Assessment On Competition 2031
Membrane Bioreactor (MBR) Systems Market: Introduction
According to the TMR report, the global MBR systems market will expand at an exponential and robust 14.5% CAGR over the period between 2021 and 2031, rising from a valuation of US$ 2,869.485 Mn in 2020 to US$ 12,725.19 Mn by 2031. Increase in demand for clean water to suffice the needs of the mounting global population and tightening regulations regarding the treatment of wastewater…
Global MBR Membrane Market Research Report 2018
Hardware, materials, items and innovations for fluid, gas or liquid cleaning, detachment and filtration are generally utilized all through the mechanical area and for private and business customers to give water, air and gas filtration and refinement abilities. Fragmentary refining (at fluid stages or cryogenic conditions), adsorption and ingestion are options in contrast to layer division of heterogeneous gas blends. Sorption techniques result in the surface amassing or modifying of…
Membrane Bioreactor (MBR) Systems Market Global Industry Analysis Forecast - 201 …
Membrane Bioreactor (MBR) is a relatively new yet fast maturing technology in the area of wastewater treatment. Prevalent technologies in wastewater treatment such as a conventional activated sludge (CAS) system are gradually replaced by MBR. Conventional systems had problems in settling the activated sludge used in the secondary clarifiers. This issue has been effectively resolved by MBR technology. Commercial MBR processes employed today utilize membranes for filtration purposes which reject…
United States MBR & UF Film Market Report 2017
MBR & UF Film Revenue, means the sales value of MBR & UF Film This report studies sales (consumption) of MBR & UF Film in United States market, focuses on the top players, with sales, price, revenue and market share for each player, covering GE Water KUBOTA Membrane Ltd. Koch Membrane Systems, Inc. Asahi Kasei Chemical Toray Chemistry, Inc. Mitsubishi Rayon Co.,LTD. Memstar Pentair Nitto Denko TOYOBO Market Segment by…